Vitamin D3 potentiates the antitumorigenic effects of arsenic trioxide in human leukemia (HL-60) cells
- PMID: 24661615
- PMCID: PMC3973008
- DOI: 10.1186/2162-3619-3-9
Vitamin D3 potentiates the antitumorigenic effects of arsenic trioxide in human leukemia (HL-60) cells
Abstract
Background: Arsenic trioxide (ATO) is a novel form of therapy that has been found to aid acute promyelocytic leukemia (APL) patients. Our laboratory has demonstrated that ATO-induced cytotoxicity in human leukemia (HL-60) cells is mediated by oxidative stress. Pro-oxidants have been known to play a role in free radical-mediated oxidative stress. Vitamin D3, (Vit D3) an active metabolite of vitamin D has been reported to inhibit the growth of number neoplasms such as prostate, breast, colorectal, leukemia, and skin cancers. The goal of the present research was to use (HL-60) cells as an in vitro test model to evaluate whether low doses of Vit D3 potentiate the toxicity of ATO and whether this toxic action is mediated via apoptotic mechanisms.
Method: HL-60 cells were treated either with a pharmacologic dose of ATO alone and with several low doses of Vit D3. Cell survival was determined by MTT assay. Cell apoptosis was measured both by flow cytometry assessment, and DNA laddering assay.
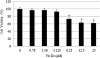
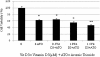
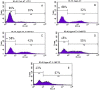
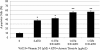
Results: MTT assay indicated that Vit D3 co-treatment potentiates ATO toxicity in HL-60 cells in a dose dependent manner. A statistically significant and dose-dependent increase (p <0.05) was recorded in annexin V positive cells (apoptotic cells) with increasing doses of Vit D3 in ATO-treated cells. This finding was confirmed by the result of DNA laddering assay showing clear evidence of nucleosomal DNA fragmentation in vitamin and ATO co-treated cells.
Conclusion: The present study indicates that Vit D3 potentiates the antitumor effects of ATO. This potentiation is mediated at least in part, through induction of phosphatidylserine externalization and nucleosomal DNA fragmentation. These findings highlight the potential impact of Vit D3 in promoting the pharmacological effect of ATO, suggesting a possible future role of Vit D3/ATO combination therapy in patients with acute promyelocytic leukemia (APL).
Figures


Similar articles
-
Basic mechanisms of arsenic trioxide (ATO)-induced apoptosis in human leukemia (HL-60) cells.J Hematol Oncol. 2010 Aug 26;3:28. doi: 10.1186/1756-8722-3-28. J Hematol Oncol. 2010. PMID: 20796291 Free PMC article.
-
In vitro assessment of oxidative stress and apoptotic mechanisms of garlic extract in the treatment of acute promyelocytic leukemia.J Cancer Sci Ther. 2012 Jan 1;2012(Suppl 3):6. doi: 10.4172/1948-5956.S3-006. J Cancer Sci Ther. 2012. PMID: 23847719 Free PMC article.
-
Differential effect of ascorbic acid and n-acetyl-L-cysteine on arsenic trioxide-mediated oxidative stress in human leukemia (HL-60) cells.J Biochem Mol Toxicol. 2008 Mar-Apr;22(2):85-92. doi: 10.1002/jbt.20223. J Biochem Mol Toxicol. 2008. PMID: 18418892 Free PMC article.
-
Arsenic trioxide in hematological malignancies: the new discovery of an ancient drug.Curr Pharm Biotechnol. 2006 Dec;7(6):397-405. doi: 10.2174/138920106779116829. Curr Pharm Biotechnol. 2006. PMID: 17168655 Review.
-
Balance between the toxicity and anticancer activity of arsenic trioxide in treatment of acute promyelocytic leukemia.Toxicol Appl Pharmacol. 2020 Dec 15;409:115299. doi: 10.1016/j.taap.2020.115299. Epub 2020 Oct 20. Toxicol Appl Pharmacol. 2020. PMID: 33091440 Review.
Cited by
-
Dual effect of oxidative stress on leukemia cancer induction and treatment.J Exp Clin Cancer Res. 2014 Dec 18;33:106. doi: 10.1186/s13046-014-0106-5. J Exp Clin Cancer Res. 2014. PMID: 25519934 Free PMC article. Review.
-
Potential association between arsenic and vitamin D.Front Endocrinol (Lausanne). 2024 Jul 17;15:1430980. doi: 10.3389/fendo.2024.1430980. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39086904 Free PMC article. No abstract available.
-
Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells.BMC Cancer. 2014 Sep 23;14:693. doi: 10.1186/1471-2407-14-693. BMC Cancer. 2014. PMID: 25245097 Free PMC article.
-
Mechanistic update of Trisenox in blood cancer.Curr Res Pharmacol Drug Discov. 2023 Nov 20;5:100166. doi: 10.1016/j.crphar.2023.100166. eCollection 2023. Curr Res Pharmacol Drug Discov. 2023. PMID: 38074774 Free PMC article. Review.
-
Proteomics-Based Identification of Differentially Abundant Proteins from Human Keratinocytes Exposed to Arsenic Trioxide.J Proteomics Bioinform. 2014 Jul;7(7):166-178. doi: 10.4172/jpb.1000317. J Proteomics Bioinform. 2014. PMID: 25419056 Free PMC article.
References
-
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. - DOI - PubMed
-
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. - PubMed
-
- Chen GQ, Zhu J, Shi XG, Ni JN, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR/PML proteins. Blood. 1996;88:1052–1061. - PubMed
-
- Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, Fermand JP. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999;59:1041–1048. - PubMed
-
- Shen L, Chen TX, Wang YP, Lin Z, Zhao HJ, Zu YZ, Wu G, Ying DM. Arsenic trioxide induced apoptosis of the human B lymphoma cell line MBC-1. J Biol Regulat Homeost Agent. 2000;14:116–119. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

