Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study
- PMID: 24661633
- PMCID: PMC4060570
- DOI: 10.1186/ar4520
Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study
Abstract
Introduction: In our present single-center pilot study, umbilical cord (UC)-derived mesenchymal stem cells (MSCs) had a good safety profile and therapeutic effect in severe and refractory systemic lupus erythematosus (SLE). The present multicenter clinical trial was undertaken to assess the safety and efficacy of allogeneic UC MSC transplantation (MSCT) in patients with active and refractory SLE.
Methods: Forty patients with active SLE were recruited from four clinical centers in China. Allogeneic UC MSCs were infused intravenously on days 0 and 7. The primary endpoints were safety profiles. The secondary endpoints included major clinical response (MCR), partial clinical response (PCR) and relapse. Clinical indices, including Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score, British Isles Lupus Assessment Group (BILAG) score and renal functional indices, were also taken into account.
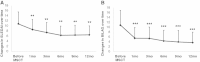
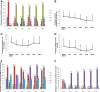
Results: The overall survival rate was 92.5% (37 of 40 patients). UC-MSCT was well tolerated, and no transplantation-related adverse events were observed. Thirteen and eleven patients achieved MCR (13 of 40, 32.5%) and PCR (11 of 40, 27.5%), respectively, during 12 months of follow up. Three and four patients experienced disease relapse at 9 months (12.5%) and 12 months (16.7%) of follow-up, respectively, after a prior clinical response. SLEDAI scores significantly decreased at 3, 6, 9 and 12 months follow-up. Total BILAG scores markedly decreased at 3 months and continued to decrease at subsequent follow-up visits. BILAG scores for renal, hematopoietic and cutaneous systems significantly improved. Among those patients with lupus nephritis, 24-hour proteinuria declined after transplantation, with statistically differences at 9 and 12 months. Serum creatinine and urea nitrogen decreased to the lowest level at 6 months, but these values slightly increased at 9 and 12 months in seven relapse cases. In addition, serum levels of albumin and complement 3 increased after MSCT, peaked at 6 months and then slightly declined by the 9- and 12-month follow-up examinations. Serum antinuclear antibody and anti-double-stranded DNA antibody decreased after MSCT, with statistically significant differences at 3-month follow-up examinations.
Conclusion: UC-MSCT results in satisfactory clinical response in SLE patients. However, in our present study, several patients experienced disease relapse after 6 months, indicating the necessity to repeat MSCT after 6 months.
Trial registry: ClinicalTrials.gov identifier: NCT01741857. Registered 26 September 2012.
Figures

Comment in
-
Safety and feasibility of umbilical cord mesenchymal stem cells in treatment-refractory systemic lupus erythematosus nephritis: time for a double-blind placebo-controlled trial to determine efficacy.Arthritis Res Ther. 2014 Jul 30;16(4):113. doi: 10.1186/ar4677. Arthritis Res Ther. 2014. PMID: 25166210 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

