Chloride channel blockers promote relaxation of TEA-induced contraction in airway smooth muscle
- PMID: 24662476
- PMCID: PMC4131261
- DOI: 10.1540/jsmr.49.112
Chloride channel blockers promote relaxation of TEA-induced contraction in airway smooth muscle
Abstract
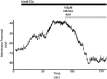
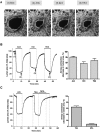
Enhanced airway smooth muscle (ASM) contraction is an important component in the pathophysiology of asthma. We have shown that ligand gated chloride channels modulate ASM contractile tone during the maintenance phase of an induced contraction, however the role of chloride flux in depolarization-induced contraction remains incompletely understood. To better understand the role of chloride flux under these conditions, muscle force (human ASM, guinea pig ASM), peripheral small airway luminal area (rat ASM) and airway smooth muscle plasma membrane electrical potentials (human cultured ASM) were measured. We found ex vivo guinea pig airway rings, human ASM strips and small peripheral airways in rat lungs slices relaxed in response to niflumic acid following depolarization-induced contraction induced by K(+) channel blockade with tetraethylammonium chloride (TEA). In isolated human airway smooth muscle cells TEA induce depolarization as measured by a fluorescent indicator or whole cell patch clamp and this depolarization was reversed by niflumic acid. These findings demonstrate that ASM depolarization induced contraction is dependent on chloride channel activity. Targeting of chloride channels may be a novel approach to relax hypercontractile airway smooth muscle in bronchoconstrictive disorders.
Figures



References
-
- Sondik EJ, Madans JH, Gentleman JF. Summary Health Statistics for U.S. Adults: National Health Interview Survey 2011, U.S. Department of Health and Human Services. Vital and Health Statistics ed. Center for Disease Prevention and Control, 2012: 1–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

