Multiple toxin-antitoxin systems in Mycobacterium tuberculosis
- PMID: 24662523
- PMCID: PMC3968373
- DOI: 10.3390/toxins6031002
Multiple toxin-antitoxin systems in Mycobacterium tuberculosis
Abstract
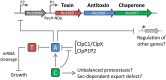
The hallmark of Mycobacterium tuberculosis is its ability to persist for a long-term in host granulomas, in a non-replicating and drug-tolerant state, and later awaken to cause disease. To date, the cellular factors and the molecular mechanisms that mediate entry into the persistence phase are poorly understood. Remarkably, M. tuberculosis possesses a very high number of toxin-antitoxin (TA) systems in its chromosome, 79 in total, regrouping both well-known (68) and novel (11) families, with some of them being strongly induced in drug-tolerant persisters. In agreement with the capacity of stress-responsive TA systems to generate persisters in other bacteria, it has been proposed that activation of TA systems in M. tuberculosis could contribute to its pathogenesis. Herein, we review the current knowledge on the multiple TA families present in this bacterium, their mechanism, and their potential role in physiology and virulence.
Figures

References
-
- Wang X., Lord D.M., Cheng H.Y., Osbourne D.O., Hong S.H., Sanchez-Torres V., Quiroga C., Zheng K., Herrmann T., Peti W., et al. A new type V toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS. Nat. Chem. Biol. 2012;8:855–861. doi: 10.1038/nchembio.1062. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

