Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold
- PMID: 24662598
- PMCID: PMC4082474
- DOI: 10.1158/1940-6207.CAPR-13-0430
Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold
Abstract
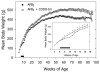
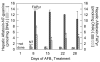
In experimental animals and humans, aflatoxin B1 (AFB1) is a potent hepatic toxin and carcinogen. The synthetic oleanane triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im), a powerful activator of Keap1-Nrf2 signaling, protects against AFB1-induced toxicity and preneoplastic lesion formation (GST-P-positive foci). This study assessed and mechanistically characterized the chemoprotective efficacy of CDDO-Im against AFB1-induced hepatocellular carcinoma (HCC). A lifetime cancer bioassay was undertaken in F344 rats dosed with AFB1 (200 μg/kg rat/day) for four weeks and receiving either vehicle or CDDO-Im (three times weekly), one week before and throughout the exposure period. Weekly, 24-hour urine samples were collected for analysis of AFB1 metabolites. In a subset of rats, livers were analyzed for GST-P foci. The comparative response of a toxicogenomic RNA expression signature for AFB1 was examined. CDDO-Im completely protected (0/20) against AFB1-induced liver cancer compared with a 96% incidence (22/23) observed in the AFB1 group. With CDDO-Im treatment, integrated level of urinary AFB1-N(7)-guanine was significantly reduced (66%) and aflatoxin-N-acetylcysteine, a detoxication product, was consistently elevated (300%) after the first AFB1 dose. In AFB1-treated rats, the hepatic burden of GST-P-positive foci increased substantially (0%-13.8%) over the four weeks, but was largely absent with CDDO-Im intervention. The toxicogenomic RNA expression signature characteristic of AFB1 was absent in the AFB1 + CDDO-Im-treated rats. The remarkable efficacy of CDDO-Im as an anticarcinogen is established even in the face of a significant aflatoxin adduct burden. Consequently, the absence of cancer requires a concept of a threshold for DNA damage for cancer development.
©2014 American Association for Cancer Research.
Figures

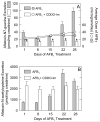
 )AFB1 or (
)AFB1 or ( ) AFB1 + CDDO-Im and 8,9-dihydro-8-(2,6-diamino-4-oxo-3,4-dihydropyrimid-5-yl formamido)-9-hydroxyaflatoxin B1 (FAPyr) receiving (
) AFB1 + CDDO-Im and 8,9-dihydro-8-(2,6-diamino-4-oxo-3,4-dihydropyrimid-5-yl formamido)-9-hydroxyaflatoxin B1 (FAPyr) receiving ( ) AFB1 or (
) AFB1 or ( ) AFB1 + CDDO-Im in DNA isolated 24 hr after the most recent dose of AFB1 over a 1 to 4 week dosing period. Values are mean ± SE (n=3-7).
) AFB1 + CDDO-Im in DNA isolated 24 hr after the most recent dose of AFB1 over a 1 to 4 week dosing period. Values are mean ± SE (n=3-7).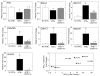
Comment in
-
Laboratory to community: chemoprevention is the answer.Cancer Prev Res (Phila). 2014 Jul;7(7):648-52. doi: 10.1158/1940-6207.CAPR-14-0124. Epub 2014 Jun 16. Cancer Prev Res (Phila). 2014. PMID: 24934618
-
Of mice, rats, and men: could Nrf2 activation protect against aflatoxin heptocarcinogenesis in humans?Cancer Prev Res (Phila). 2014 Jul;7(7):653-7. doi: 10.1158/1940-6207.CAPR-14-0119. Epub 2014 Jun 16. Cancer Prev Res (Phila). 2014. PMID: 24934619
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J. Cancer. 2010;127:2893–2917. - PubMed
-
- Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, Hall AJ, et al. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in West Africa: A community-based intervention study. Lancet. 2005;365:1950–1956. - PubMed
-
- Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): Predictive role for short-term molecular dosimetry. Cancer Res. 1991;51:5501–5506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

