A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering
- PMID: 24662725
- PMCID: PMC3964514
- DOI: 10.1038/srep04457
A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering
Abstract
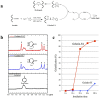
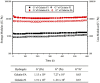
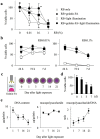
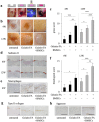
Osteochondral injuries remain difficult to repair. We developed a novel photo-cross-linkable furfurylamine-conjugated gelatin (gelatin-FA). Gelatin-FA was rapidly cross-linked by visible light with Rose Bengal, a light sensitizer, and was kept gelled for 3 weeks submerged in saline at 37°C. When bone marrow-derived stromal cells (BMSCs) were suspended in gelatin-FA with 0.05% Rose Bengal, approximately 87% of the cells were viable in the hydrogel at 24 h after photo-cross-linking, and the chondrogenic differentiation of BMSCs was maintained for up to 3 weeks. BMP4 fusion protein with a collagen binding domain (CBD) was retained in the hydrogels at higher levels than unmodified BMP4. Gelatin-FA was subsequently employed as a scaffold for BMSCs and CBD-BMP4 in a rabbit osteochondral defect model. In both cases, the defect was repaired with articular cartilage-like tissue and regenerated subchondral bone. This novel, photo-cross-linkable gelatin appears to be a promising scaffold for the treatment of osteochondral injury.
Figures


References
-
- Buckwalter J. A., Mankin H. J. & Grodzinsky A. J. Articular cartilage and osteoarthritis. Instr Course Lect 54, 465–480 (2005). - PubMed
-
- Mandelbaum B. R. et al. Articular cartilage lesions of the knee. Am J Sports Med 26, 853–861 (1998). - PubMed
-
- Buckwalter J. A., Martin J. A. & Brown T. D. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43, 603–609 (2006). - PubMed
-
- Nukavarapu S. P. & Dorcemus D. L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol Adv 31, 706–721 (2013). - PubMed
-
- Panseri S. et al. Osteochondral tissue engineering approaches for articular cartilage and subchondral bone regeneration. Knee Surg Sports Traumatol Arthrosc 20, 1182–1191 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

