Muscarinic acetylcholine receptor X-ray structures: potential implications for drug development
- PMID: 24662799
- PMCID: PMC4065632
- DOI: 10.1016/j.coph.2014.02.006
Muscarinic acetylcholine receptor X-ray structures: potential implications for drug development
Abstract
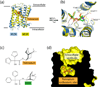
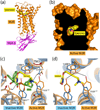
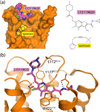
Muscarinic acetylcholine receptor antagonists are widely used as bronchodilating drugs in pulmonary medicine. The therapeutic efficacy of these agents depends on the blockade of M3 muscarinic receptors expressed on airway smooth muscle cells. All muscarinic antagonists currently used as bronchodilating agents show high affinity for all five muscarinic receptor subtypes, thus increasing the likelihood of unwanted side effects. Recent X-ray crystallographic studies have provided detailed structural information about the nature of the orthosteric muscarinic binding site (the conventional acetylcholine binding site) and an 'outer' receptor cavity that can bind allosteric (non-orthosteric) drugs. These new findings should guide the development of selective M3 receptor blockers that have little or no effect on other muscarinic receptor subtypes.
Published by Elsevier Ltd.
Figures
References
-
- Chapter 36. Pulmonary Pharmacology. In: Barnes PJ, editor; Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. http://wwwaccessmedicinecom/resourceTOCaspx?resourceID=651.
-
- Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504. - PubMed
-
- Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003;98:59–69. - PubMed
-
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

