Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes
- PMID: 24662828
- PMCID: PMC4162459
- DOI: 10.1038/onc.2014.66
Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes
Abstract
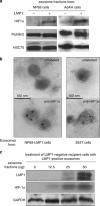
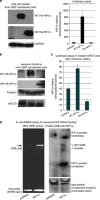
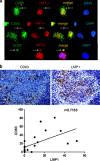
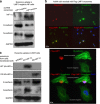
It has emerged recently that exosomes are potential carriers of pro-tumorigenic factors that participate in oncogenesis. However, whether oncogenic transcription factors are transduced by exosomes is unknown. Hypoxia-inducible factor-1α (HIF1α) transcriptionally regulates numerous key aspects of tumor development and progression by promoting a more aggressive tumor phenotype, characterized by increased proliferation and invasiveness coupled with neoangiogenesis. It has been shown that the principal oncoprotein of Epstein-Barr virus (EBV), latent membrane protein 1 (LMP1), drives oncogenic processes and tumor progression of the highly invasive EBV malignancy, nasopharyngeal carcinoma (NPC). We now demonstrate that endogenous HIF1α is detectable in exosomes and that LMP1 significantly increases levels of HIF1α in exosomes. HIF1 recovered from exosomes retains DNA-binding activity and is transcriptionally active in recipient cells after exosome uptake. We also show that treatment of EBV-negative cells with LMP1-exosomes increases migration and invasiveness of NP cell lines in functional assays, which correlates with the phenotype associated with epithelial-mesenchymal transition (EMT). In addition, we provide evidence that HIF1α itself participates in exosome-mediated pro-metastatic effects in recipient cells, as exosome-mediated delivery of active and inactive forms of HIF1α results in reciprocal changes in the expression of E- and N-cadherins associated with EMT. Further, immunohistochemical analysis of NPC tumor tissues revealed direct correlation between protein levels of LMP1 and of the endosome/exosome marker tetraspanin, CD63, which suggests an increase in exosome formation in this EBV-positive malignancy. We hypothesize that exosome-mediated transfer of functional pro-metastatic factors by LMP1-positive NPC cells to surrounding tumor cells promotes cancer progression.
Figures
References
-
- Yoshizaki T, Wakisaka N, Pagano JS.Epstein-Barr virus invasion and metastasis. In: Robertson Erle S (ed).Epstein-Barr Virus Caister Academic Press: Philadelphia, PA, USA; 2005171–196.
-
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. - PubMed
-
- Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

