Time-lapse imaging as a tool to investigate contractility of the epididymal duct--effects of cGMP signaling
- PMID: 24662987
- PMCID: PMC3963912
- DOI: 10.1371/journal.pone.0092603
Time-lapse imaging as a tool to investigate contractility of the epididymal duct--effects of cGMP signaling
Abstract
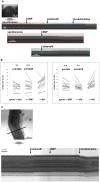
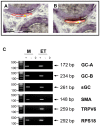
The well orchestrated function of epididymal smooth muscle cells ensures transit of spermatozoa through the epididymal duct during which spermatozoa acquire motility and fertilizing capacity. Relaxation of smooth muscle cells is mediated by cGMP signaling and components of this pathway are found within the male reproductive tract. Whereas contractile function of caudal parts of the rat epididymal duct can be examined in organ bath studies, caput and corpus regions are fragile and make it difficult to mount them in an organ bath. We developed an ex vivo time-lapse imaging-based approach to investigate the contractile pattern in these parts of the epididymal duct. Collagen-embedding allowed immobilization without impeding contractility or diffusion of drugs towards the duct and therefore facilitated subsequent movie analyses. The contractile pattern was made visible by placing virtual sections through the acquired image stack to track wall movements over time. By this, simultaneous evaluation of contractile activity at different positions of the observed duct segment was possible. With each contraction translating into a spike, drug-induced alterations in contraction frequency could be assessed easily. Peristaltic contractions were also detectable and throughout all regions in the proximal epididymis we found regular spontaneous contractile activity that elicited movement of intraluminal contents. Stimulating cGMP production by natriuretic peptide ANP or inhibiting degradation of cGMP by the phosphodiesterase 5 inhibitor sildenafil significantly reduced contractile frequency in isolated duct segments from caput and corpus. RT-PCR analysis after laser-capture microdissection localized the corresponding molecules to the smooth muscle layer of the duct. Our time-lapse imaging approach proved to be feasible to assess contractile function in all regions of the epididymal duct under near physiological conditions and provides a tool to evaluate acute (side) effects of drugs and to investigate various signaling pathways.
Conflict of interest statement
Figures




Similar articles
-
Phosphodiesterase 5 (PDE5) inhibition, ANP and NO rapidly reduce epididymal duct contractions, but long-term PDE5 inhibition in vivo does not.Mol Cell Endocrinol. 2012 Feb 26;349(2):145-53. doi: 10.1016/j.mce.2011.09.039. Epub 2011 Oct 2. Mol Cell Endocrinol. 2012. PMID: 21996373
-
Regulation of spontaneous contractile activity in the bovine epididymal duct by cyclic guanosine 5'-monophosphate-dependent pathways.Endocrinology. 2006 Apr;147(4):2051-62. doi: 10.1210/en.2005-1324. Epub 2006 Jan 26. Endocrinology. 2006. PMID: 16439452
-
Erg K+ channels modulate contractile activity in the bovine epididymal duct.Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R895-904. doi: 10.1152/ajpregu.00521.2007. Epub 2008 Jan 9. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18184764
-
Contractility of the epididymal duct: function, regulation and potential drug effects.Reproduction. 2018 Oct 1;156(4):R125-R141. doi: 10.1530/REP-17-0754. Reproduction. 2018. PMID: 30304934 Review.
-
Role of exosomes in sperm maturation during the transit along the male reproductive tract.Blood Cells Mol Dis. 2005 Jul-Aug;35(1):1-10. doi: 10.1016/j.bcmd.2005.03.005. Blood Cells Mol Dis. 2005. PMID: 15893944 Review.
Cited by
-
Gubernaculum and Epididymo-Testicular Descent: Review of the Literature.Acta Med Litu. 2022;29(2):201-210. doi: 10.15388/Amed.2022.29.2.6. Epub 2022 Jun 29. Acta Med Litu. 2022. PMID: 37733393 Free PMC article. Review.
-
Collective cell dynamics and luminal fluid flow in the epididymis: A mechanobiological perspective.Andrology. 2024 Jul;12(5):939-948. doi: 10.1111/andr.13490. Epub 2023 Jul 17. Andrology. 2024. PMID: 37415418 Free PMC article. Review.
-
Optical coherence tomography for dynamic investigation of mammalian reproductive processes.Mol Reprod Dev. 2023 Jan;90(1):3-13. doi: 10.1002/mrd.23665. Epub 2022 Dec 27. Mol Reprod Dev. 2023. PMID: 36574640 Free PMC article. Review.
-
In vivo dynamic volumetric imaging of mouse testis and epididymis with optical coherence tomography†.Biol Reprod. 2024 Feb 10;110(2):365-376. doi: 10.1093/biolre/ioad158. Biol Reprod. 2024. PMID: 37971359 Free PMC article.
-
Insights on Platelet-Derived Growth Factor Receptor α-Positive Interstitial Cells in the Male Reproductive Tract.Int J Mol Sci. 2024 Apr 8;25(7):4128. doi: 10.3390/ijms25074128. Int J Mol Sci. 2024. PMID: 38612936 Free PMC article. Review.
References
-
- Cosentino MJ, Cockett AT (1986) Structure and function of the epididymis. Urol Res 14(5): 229–240. - PubMed
-
- Robaire B, Hinton BT, Orgebin-Crist MC (2006) The epididymis. In: Neil J (ed), Knobile and Neill's Physiology of Reproduction, 3rd ed, San Diego CA, Elsevier 1071–1148.
-
- Ricker DD (1998) The autonomic innervation of the epididymis: its effects on epididymal function and fertility. J Androl 19(1): 1–4. - PubMed
-
- Ricker DD, Chang TS (1996) Neuronal input from the inferior mesenteric ganglion (IMG) affects sperm transport within the rat cauda epididymis. Int J Androl 19(6): 371–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

