Laboratory detection of artemisinin-resistant Plasmodium falciparum
- PMID: 24663013
- PMCID: PMC4068498
- DOI: 10.1128/AAC.01924-13
Laboratory detection of artemisinin-resistant Plasmodium falciparum
Abstract
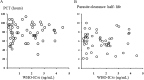
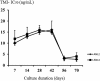
Conventional 48-h in vitro susceptibility tests have low sensitivity in identifying artemisinin-resistant Plasmodium falciparum, defined phenotypically by low in vivo parasite clearance rates. We hypothesized originally that this discrepancy was explained by a loss of ring-stage susceptibility and so developed a simple field-adapted 24-h trophozoite maturation inhibition (TMI) assay focusing on the ring stage and compared it to the standard 48-h schizont maturation inhibition (WHO) test. In Pailin, western Cambodia, where artemisinin-resistant P. falciparum is prevalent, the TMI test mean (95% confidence interval) 50% inhibitory concentration (IC50) for artesunate was 6.8 (5.2 to 8.3) ng/ml compared with 1.5 (1.2 to 1.8) ng/ml for the standard 48-h WHO test (P = 0.001). TMI IC50s correlated significantly with the in vivo responses to artesunate (parasite clearance time [r = 0.44, P = 0.001] and parasite clearance half-life [r = 0.46, P = 0.001]), whereas the standard 48-h test values did not. On continuous culture of two resistant isolates, the artemisinin-resistant phenotype was lost after 6 weeks (IC50s fell from 10 and 12 ng/ml to 2.7 and 3 ng/ml, respectively). Slow parasite clearance in falciparum malaria in western Cambodia results from reduced ring-stage susceptibility.
Copyright © 2014 Chotivanich et al.
Figures

References
-
- World Health Organization. 2010. Guidelines for the treatment of malaria, 2nd ed, p 13–47 WC 770. World Health Organization Press, Geneva, Switzerland
-
- Nosten F, White NJ. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77(6 Suppl):181–192 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

