Pharmacokinetics of rifampin and isoniazid in tuberculosis-HIV-coinfected patients receiving nevirapine- or efavirenz-based antiretroviral treatment
- PMID: 24663014
- PMCID: PMC4068429
- DOI: 10.1128/AAC.02379-13
Pharmacokinetics of rifampin and isoniazid in tuberculosis-HIV-coinfected patients receiving nevirapine- or efavirenz-based antiretroviral treatment
Abstract
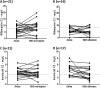
This is a substudy of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) Comparison of Nevirapine and Efavirenz for the Treatment of HIV-TB Co-infected Patients (ANRS 12146-CARINEMO) trial, which assessed the pharmacokinetics of rifampin or isoniazid with or without the coadministration of nonnucleoside reverse transcriptase inhibitor-based HIV antiretroviral therapy in HIV-tuberculosis-coinfected patients in Mozambique. Thirty-eight patients on antituberculosis therapy based on rifampin and isoniazid participated in the substudy (57.9% males; median age, 33 years; median weight, 51.9 kg; median CD4(+) T cell count, 104 cells/μl; median HIV-1 RNA load, 5.5 log copies/ml). The daily doses of rifampin and isoniazid were 10 and 5 mg/kg of body weight, respectively. Twenty-one patients received 200 mg of nevirapine twice a day (b.i.d.), and 17 patients received 600 mg of efavirenz once a day (q.d.) in combination with lamivudine and stavudine from day 1 until the end of the study. Blood samples were collected at regular time-dosing intervals after morning administration of a fixed-dose combination of rifampin and isoniazid. When rifampin was administered alone, the median maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve (AUC) at steady state were 6.59 mg/liter (range, 2.70 to 14.07 mg/liter) and 27.69 mg · h/liter (range, 11.41 to 109.75 mg · h/liter), respectively. Concentrations remained unchanged when rifampin was coadministered with nevirapine or efavirenz. When isoniazid was administered alone, the median isoniazid Cmax and AUC at steady state were 5.08 mg/liter (range, 1.26 to 11.51 mg/liter) and 20.92 mg · h/liter (range, 7.73 to 56.95 mg · h/liter), respectively. Concentrations remained unchanged when isoniazid was coadministered with nevirapine; however, a 29% decrease in the isoniazid AUC was observed when isoniazid was combined with efavirenz. The pharmacokinetic parameters of rifampin and isoniazid when coadministered with nevirapine or efavirenz were not altered to a clinically significant extent in these severely immunosuppressed HIV-infected patients. Patients experienced favorable clinical outcomes. (This study has been registered at ClinicalTrials.gov under registration no. NCT00495326.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- World Health Organization. 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/
-
- Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. 2010. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 362:697–706. 10.1056/NEJMoa0905848 - DOI - PMC - PubMed
-
- World Health Organization. 2010. Guidelines for treatment of tuberculosis, fourth edition World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2010/9789241547833/en/index.html
-
- Barry C, Waring J, Stapledon R, Konstantinos A, National Tuberculosis Advisory Committee, for the Communicable Diseases Network Australia 2012. Tuberculosis notifications in Australia, 2008 and 2009. Commun. Dis. Intell. Q. Rep. 36:82–94 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

