Preclinical profile of BI 224436, a novel HIV-1 non-catalytic-site integrase inhibitor
- PMID: 24663024
- PMCID: PMC4068430
- DOI: 10.1128/AAC.02719-13
Preclinical profile of BI 224436, a novel HIV-1 non-catalytic-site integrase inhibitor
Abstract
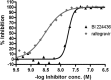
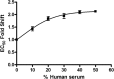
BI 224436 is an HIV-1 integrase inhibitor with effective antiviral activity that acts through a mechanism that is distinct from that of integrase strand transfer inhibitors (INSTIs). This 3-quinolineacetic acid derivative series was identified using an enzymatic integrase long terminal repeat (LTR) DNA 3'-processing assay. A combination of medicinal chemistry, parallel synthesis, and structure-guided drug design led to the identification of BI 224436 as a candidate for preclinical profiling. It has antiviral 50% effective concentrations (EC50s) of <15 nM against different HIV-1 laboratory strains and cellular cytotoxicity of >90 μM. BI 224436 also has a low, ∼2.1-fold decrease in antiviral potency in the presence of 50% human serum and, by virtue of a steep dose-response curve slope, exhibits serum-shifted EC95 values ranging between 22 and 75 nM. Passage of virus in the presence of inhibitor selected for either A128T, A128N, or L102F primary resistance substitutions, all mapping to a conserved allosteric pocket on the catalytic core of integrase. BI 224436 also retains full antiviral activity against recombinant viruses encoding INSTI resistance substitutions N155S, Q148H, and E92Q. In drug combination studies performed in cellular antiviral assays, BI 224436 displays an additive effect in combination with most approved antiretrovirals, including INSTIs. BI 224436 has drug-like in vitro absorption, distribution, metabolism, and excretion (ADME) properties, including Caco-2 cell permeability, solubility, and low cytochrome P450 inhibition. It exhibited excellent pharmacokinetic profiles in rat (clearance as a percentage of hepatic flow [CL], 0.7%; bioavailability [F], 54%), monkey (CL, 23%; F, 82%), and dog (CL, 8%; F, 81%). Based on the excellent biological and pharmacokinetic profile, BI 224436 was advanced into phase 1 clinical trials.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Llano M, Vanegas M, Fregoso O, Saenz D, Chung S, Peretz M, Poeschla EM. 2004. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J. Virol. 78:9524–9537. 10.1128/JVI.78.17.9524-9537.2004 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

