Inhibitors of the tick-borne, hemorrhagic fever-associated flaviviruses
- PMID: 24663025
- PMCID: PMC4068503
- DOI: 10.1128/AAC.02393-14
Inhibitors of the tick-borne, hemorrhagic fever-associated flaviviruses
Abstract
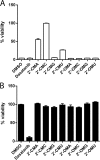
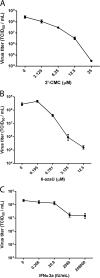
No antiviral therapies are available for the tick-borne flaviviruses associated with hemorrhagic fevers: Kyasanur Forest disease virus (KFDV), both classical and the Alkhurma hemorrhagic fever virus (AHFV) subtype, and Omsk hemorrhagic fever virus (OHFV). We tested compounds reported to have antiviral activity against members of the Flaviviridae family for their ability to inhibit AHFV replication. 6-Azauridine (6-azaU), 2'-C-methylcytidine (2'-CMC), and interferon alpha 2a (IFN-α2a) inhibited the replication of AHFV and also KFDV, OHFV, and Powassan virus. The combination of IFN-α2a and 2'-CMC exerted an additive antiviral effect on AHFV, and the combination of IFN-α2a and 6-azaU was moderately synergistic. The combination of 2'-CMC and 6-azaU was complex, being strongly synergistic but with a moderate level of antagonism. The antiviral activity of 6-azaU was reduced by the addition of cytidine but not guanosine, suggesting that it acted by inhibiting pyrimidine biosynthesis. To investigate the mechanism of action of 2'-CMC, AHFV variants with reduced susceptibility to 2'-CMC were selected. We used a replicon system to assess the substitutions present in the selected AHFV population. A double NS5 mutant, S603T/C666S, and a triple mutant, S603T/C666S/M644V, were more resistant to 2'-CMC than the wild-type replicon. The S603T/C666S mutant had a reduced level of replication which was increased when M644V was also present, although the replication of this triple mutant was still below that of the wild type. The S603 and C666 residues were predicted to lie in the active site of the AHFV NS5 polymerase, implicating the catalytic center of the enzyme as the binding site for 2'-CMC.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Gubler DJ, Kuno G, Markhoff L. 2007. Flaviviruses. In Knipe DM, Howley PM. (ed), Fields virology 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

