Physicians' attitudes about multiplex tumor genomic testing
- PMID: 24663044
- PMCID: PMC3992721
- DOI: 10.1200/JCO.2013.52.4298
Physicians' attitudes about multiplex tumor genomic testing
Abstract
Purpose: Although predictive multiplex somatic genomic tests hold the potential to transform care by identifying targetable alterations in multiple cancer genes, little is known about how physicians will use such tests in practice.
Participants and methods: Before the initiation of enterprise-wide multiplex testing at a major cancer center, we surveyed all clinically active adult cancer physicians to assess their current use of somatic testing, their attitudes about multiplex testing, and their genomic confidence.
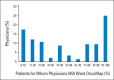
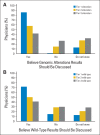
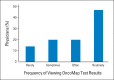
Results: A total of 160 physicians participated (response rate, 61%): 57% were medical oncologists; 29%, surgeons; 14% radiation oncologists; 37%, women; and 83%, research principal investigators. Twenty-two percent of physicians reported low confidence in their genomic knowledge. Eighteen percent of physicians anticipated testing patients infrequently (≤ 10%), whereas 25% anticipate testing most patients (≥ 90%). Higher genomic confidence was associated with wanting to test a majority of patients (adjusted odds ratio [OR], 6.09; 95% CI, 2.1 to 17.5) and anticipating using actionable (adjusted OR, 2.46; 95% CI, 1.2 to 5.2) or potentially actionable (adjusted OR, 2.89; 95% CI, 1.1 to 7.9) test results to inform treatment recommendations. Forty-two percent of physicians endorsed disclosure of uncertain genomic findings to patients.
Conclusion: Physicians at a tertiary-care National Cancer Institute-designated comprehensive cancer center varied considerably in how they planned to incorporate predictive multiplex somatic genomic tests into practice and in their attitudes about the disclosure of genomic information of uncertain significance. Given that many physicians reported low genomic confidence, evidence-based guidelines and enhanced physician genomic education efforts may be needed to ensure that genomically guided cancer care is adequately delivered.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Comment in
-
Conflicted confidence: academic oncologists' views on multiplex pharmacogenomic testing.J Clin Oncol. 2014 May 1;32(13):1290-2. doi: 10.1200/JCO.2013.54.8016. Epub 2014 Mar 24. J Clin Oncol. 2014. PMID: 24663051 No abstract available.
References
-
- Garraway LA. Genomics-driven oncology: Framework for an emerging paradigm. J Clin Oncol. 2013;31:1806–1814. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
-
- Ciardiello F, Tejpar S, Normanno N, et al. Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol. 2011;6:133–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

