No stress! Relax! Mechanisms governing growth and shape in plant cells
- PMID: 24663059
- PMCID: PMC3975442
- DOI: 10.3390/ijms15035094
No stress! Relax! Mechanisms governing growth and shape in plant cells
Abstract
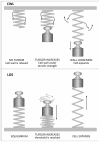
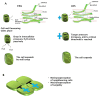
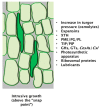
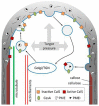
The mechanisms through which plant cells control growth and shape are the result of the coordinated action of many events, notably cell wall stress relaxation and turgor-driven expansion. The scalar nature of turgor pressure would drive plant cells to assume spherical shapes; however, this is not the case, as plant cells show an amazing variety of morphologies. Plant cell walls are dynamic structures that can display alterations in matrix polysaccharide composition and concentration, which ultimately affect the wall deformation rate. The wide varieties of plant cell shapes, spanning from elongated cylinders (as pollen tubes) and jigsaw puzzle-like epidermal cells, to very long fibres and branched stellate leaf trichomes, can be understood if the underlying mechanisms regulating wall biosynthesis and cytoskeletal dynamics are addressed. This review aims at gathering the available knowledge on the fundamental mechanisms regulating expansion, growth and shape in plant cells by putting a special emphasis on the cell wall-cytoskeleton system continuum. In particular, we discuss from a molecular point of view the growth mechanisms characterizing cell types with strikingly different geometries and describe their relationship with primary walls. The purpose, here, is to provide the reader with a comprehensive overview of the multitude of events through which plant cells manage to expand and control their final shapes.
Figures


References
-
- Geitmann A., Ortega J.K. Mechanics and modeling of plant cell growth. Trends Plant Sci. 2009;1:467–478. - PubMed
-
- Cosgrove D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. - PubMed
-
- Humphrey T.V., Bonetta D.T., Goring D.R. Sentinels at the wall: Cell wall receptors and sensors. New Phytol. 2007;176:7–21. - PubMed
-
- Hamann T., Bennett M., Mansfield J., Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009;57:1015–1026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

