New and rare carotenoids isolated from marine bacteria and their antioxidant activities
- PMID: 24663119
- PMCID: PMC3967232
- DOI: 10.3390/md12031690
New and rare carotenoids isolated from marine bacteria and their antioxidant activities
Abstract
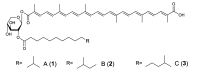
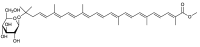
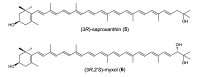
Marine bacteria have not been examined as extensively as land bacteria. We screened carotenoids from orange or red pigments-producing marine bacteria belonging to rare or novel species. The new acyclic carotenoids with a C₃₀ aglycone, diapolycopenedioc acid xylosylesters A-C and methyl 5-glucosyl-5,6-dihydro-apo-4,4'-lycopenoate, were isolated from the novel Gram-negative bacterium Rubritalea squalenifaciens, which belongs to phylum Verrucomicrobia, as well as the low-GC Gram-positive bacterium Planococcus maritimus strain iso-3 belonging to the class Bacilli, phylum Firmicutes, respectively. The rare monocyclic C₄₀ carotenoids, (3R)-saproxanthin and (3R,2'S)-myxol, were isolated from novel species of Gram-negative bacteria belonging to the family Flavobacteriaceae, phylum Bacteroidetes. In this review, we report the structures and antioxidant activities of these carotenoids, and consider relationships between bacterial phyla and carotenoid structures.
Figures
References
-
- Britton G., Liaaen-Jensen S., Pfander H. Carotenoids Handbook. Birkhäuser Verlag; Basel, Switzerland: 2004.
-
- Iwamoto T., Hosoda K., Hirano R., Kurata H., Matsumoto A., Miki W., Kamiyama M., Itakura H., Yamamoto S., Kondo K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheoscler. Thomb. 2000;7:216–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous