Phosphine catalysis of allenes with electrophiles
- PMID: 24663290
- PMCID: PMC4059616
- DOI: 10.1039/c4cs00054d
Phosphine catalysis of allenes with electrophiles
Abstract
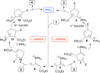
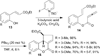
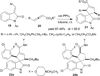
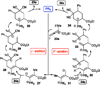
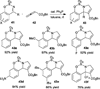
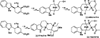
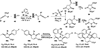
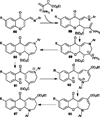
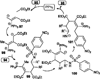
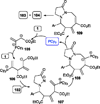
Nucleophilic phosphine catalysis of allenes with electrophiles is one of the most powerful and straightforward synthetic strategies for the generation of highly functionalized carbocycle or heterocycle structural motifs, which are present in a wide range of bioactive natural products and medicinally important substances. The reaction topologies can be controlled through a judicious choice of the phosphine catalyst and the structural variations of starting materials. This Tutorial Review presents selected examples of nucleophilic phosphine catalysis using allenes and electrophiles.
Figures




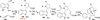
References
-
- Valentine DH, Hillhouse JH. Synthesis. 2003;317
-
- Quin LD, editor. A Guide to Organophosphorus Chemistry. New York: Wiley; 2000.
-
- Rauhut MM, H Currier. U. S. Patent 3 074 999, 1963; Chem. Abstr. 1963;58:11224a.
-
- Winterfeldt E, Dillinger HJ. Chem. Ber. 1966;99:1558.
-
- Morita K, Suzuki Z, Hirose H. Bull. Chem. Soc. Jpn. 1968;41:2815.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

