Heart failure with preserved ejection fraction
- PMID: 24663384
- PMCID: PMC4075067
- DOI: 10.1007/s00424-014-1480-8
Heart failure with preserved ejection fraction
Abstract
As part of this series devoted to heart failure (HF), we review the epidemiology, diagnosis, pathophysiology, and treatment of HF with preserved ejection fraction (HFpEF). Gaps in knowledge and needed future research are discussed.
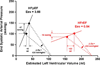
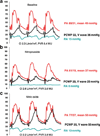
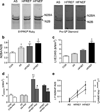
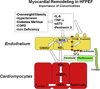
Figures





References
-
- Andersen MJ, Ersboll M, Axelsson A, Gustafsson F, Hassager C, Kober L, Borlaug BA, Boesgaard S, Skovgaard LT, Moller JE. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: the Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) trial. Circulation. 2013;127:1200–1208. - PubMed
-
- Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or =40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–209. - PubMed
-
- Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

