Frequent complications and severe bone loss associated with the repiphysis expandable distal femoral prosthesis
- PMID: 24664193
- PMCID: PMC4317465
- DOI: 10.1007/s11999-014-3564-3
Frequent complications and severe bone loss associated with the repiphysis expandable distal femoral prosthesis
Abstract
Background: The treatment of choice for distal femur malignancies in skeletally immature patients remains controversial. An expandable endoprosthesis device (Repiphysis Limb Salvage System; Wright Medical Technology, Arlington, TN, USA) allows for limb preservation and noninvasive lengthening but has been associated with significant complications; however, the extent and implications of bone loss associated with this implant have not been reported.
Questions/purposes: Our goals were to report (1) the 2-year minimum clinical outcomes after placement of the Repiphysis expandable prosthesis for pediatric distal femur malignancies; (2) the complications associated with this prosthesis; (3) the failure rate of this prosthesis; and (4) the revision alternatives available for salvage procedures.
Methods: Between 2002 and 2010, one surgeon (SG) treated all skeletally immature patients (mean age, 10.1 years; range, 4.7-13.6 years) with distal femoral osteosarcoma using a Repiphysis expandable prosthesis. Of the 12 patients who met these criteria, two were excluded for death from disease before 2 years, and mean followup for the remaining 10 was 72 months (range, 26-119 months). Medical records were retrospectively reviewed for complications and clinical outcomes, as assessed by the Musculoskeletal Tumor Society (MSTS) scoring system. Radiographs at final followup were reviewed for bone loss and analyzed by the two senior authors (SG, WWV) to determine reconstruction options available for future revisions.
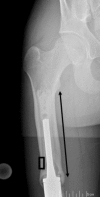
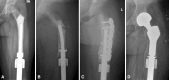
Results: MSTS scores averaged 67%, and we observed 37 implant-related complications requiring a total of 15 reoperations. Six patients underwent implant revisions with aseptic loosening being the predominant mode of failure; ultimately, four of these were converted to adult modular oncology prostheses, and two underwent total femoral replacements. Bone loss in this series was severe in terms of femoral length, cortical thinning, and metadiaphyseal compromise, and most patients will not have sufficient bone stock to permit future revision using standard stem fixation.
Conclusions: The bone loss around the stem of this prosthesis limits subsequent revision options, often resulting in a total femoral prosthesis. Although the decision to use the Repiphysis device must be made on an individual basis, surgeons should recognize the potential for significant bone compromise limiting revision options and consider other options.
Level of evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Figures

References
-
- Beebe K, Benevenia J, Kaushal N, Uglialoro A, Patel N, Patterson F. Evaluation of a noninvasive expandable prosthesis in musculoskeletal oncology patients for the upper and lower limb. Orthopedics. 2010;33:396. - PubMed
-
- DiCaprio MR, Friedlaender GE. Malignant bone tumors: limb sparing versus amputation. J Am Acad Orthop Surg. 2003;11:25–37. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

