Endoplasmic reticulum stress-mediated activation of p38 MAPK, Caspase-2 and Caspase-8 leads to abrin-induced apoptosis
- PMID: 24664279
- PMCID: PMC3963924
- DOI: 10.1371/journal.pone.0092586
Endoplasmic reticulum stress-mediated activation of p38 MAPK, Caspase-2 and Caspase-8 leads to abrin-induced apoptosis
Abstract
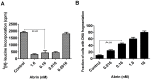
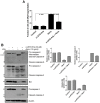
Abrin from Abrus precatorius plant is a potent protein synthesis inhibitor and induces apoptosis in cells. However, the relationship between inhibition of protein synthesis and apoptosis is not well understood. Inhibition of protein synthesis by abrin can lead to accumulation of unfolded protein in the endoplasmic reticulum causing ER stress. The observation of phosphorylation of eukaryotic initiation factor 2α and upregulation of CHOP (CAAT/enhancer binding protein (C/EBP) homologous protein), important players involved in ER stress signaling by abrin, suggested activation of ER stress in the cells. ER stress is also known to induce apoptosis via stress kinases such as p38 MAPK and JNK. Activation of both the pathways was observed upon abrin treatment and found to be upstream of the activation of caspases. Moreover, abrin-induced apoptosis was found to be dependent on p38 MAPK but not JNK. We also observed that abrin induced the activation of caspase-2 and caspase-8 and triggered Bid cleavage leading to mitochondrial membrane potential loss and thus connecting the signaling events from ER stress to mitochondrial death machinery.
Conflict of interest statement
Figures






References
-
- Hartley MR, Lord JM (2004) Cytotoxic ribosome-inactivating lectins from plants. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1701: 1–14. - PubMed
-
- Nielsen K, Boston RS (2001) Ribosome-inactivating proteins: A plant perspective. Annual Review of Plant Physiology and Plant Molecular Biology 52: 785–816. - PubMed
-
- Peumans WJ, Hao Q, Van Damme EJM (2001) Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? The FASEB Journal 15: 1493–1506. - PubMed
-
- Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, et al. (1996) Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer 68: 349–355. - PubMed
-
- Narayanan S, Surendranath K, Bora N, Surolia A, Karande AA (2005) Ribosome inactivating proteins and apoptosis. FEBS Letters 579: 1324–1331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

