Systemic corticosteroids for acute sinusitis
- PMID: 24664368
- PMCID: PMC11179165
- DOI: 10.1002/14651858.CD008115.pub3
Systemic corticosteroids for acute sinusitis
Abstract
Background: Acute sinusitis is the inflammation and swelling of the nasal and paranasal mucous membranes and is a common reason for patients to seek primary care consultations. The related impairment of daily functioning and quality of life is attributable to symptoms such as facial pain and nasal congestion.
Objectives: To assess the effects of systemic corticosteroids on clinical response rates and to determine adverse effects and relapse rates of systemic corticosteroids compared to placebo or standard clinical care in children and adults with acute sinusitis.
Search methods: We searched CENTRAL (2014, Issue 1), MEDLINE (1966 to February week 1, 2014) and EMBASE (January 2009 to February 2014).
Selection criteria: Randomised controlled trials (RCTs) comparing systemic corticosteroids to placebo or standard clinical care for patients with acute sinusitis.
Data collection and analysis: Two review authors independently assessed the methodological quality of the trials and extracted data.
Main results: Five RCTs with a total of 1193 adult participants met our inclusion criteria. We judged methodological quality to be moderate in four trials and high in one trial. Acute sinusitis was defined clinically in all trials. However, the three trials performed in ear, nose and throat (ENT) outpatient clinics also used radiological assessment as part of their inclusion criteria. All participants were assigned to either oral corticosteroids (prednisone 24 mg to 80 mg daily or betamethasone 1 mg daily) or the control treatment (placebo in four trials and non-steroidal anti-inflammatory drugs (NSAIDs) in one trial). In four trials antibiotics were prescribed in addition to oral corticosteroids or control treatment, while one trial investigated the effects of oral corticosteroids as a monotherapy.When combining data from the five trials, participants treated with oral corticosteroids were more likely to have short-term resolution or improvement of symptoms than those receiving the control treatment: at days three to seven (risk ratio (RR) 1.3, 95% confidence interval (CI) 1.1 to 1.6; risk difference (RD) 17%, 95% CI 6% to 29%) and at days four to 14 (RR 1.2, 95% CI 1.0 to 1.5; RD 14%, 95% CI 1% to 27%). A sensitivity analysis including the four trials with placebo as a control treatment showed similar results but with a lesser effect size: at days three to seven (RR 1.2, 95% CI 1.1 to 1.3; RD 11%, 95% CI 4% to 17%) and days four to 14 (RR 1.1, 95% CI 1.0 to 1.2; RD 8%, 95% CI 2% to 13%). Statistical heterogeneity was high for many analyses. Subgroup analyses revealed that corticosteroid monotherapy had no beneficial effects. Furthermore, scenario analysis showed that outcomes missing from the trial reports might have introduced attrition bias (a worst-case scenario showed no statistically significant beneficial effect of oral corticosteroids). No trial reported effects on relapse or recurrence rates. Reported side effects in patients treated with oral corticosteroids were mild (nausea, vomiting, gastric complaints) and did not significantly differ from those receiving placebo.
Authors' conclusions: Oral corticosteroids as a monotherapy appear to be ineffective for adult patients with clinically diagnosed acute sinusitis. Current data on the use of oral corticosteroids as an adjunctive therapy to oral antibiotics are limited: almost all trials are performed in secondary care settings and there is a significant risk of bias. This limited evidence suggests that oral corticosteroids in combination with antibiotics may be modestly beneficial for short-term relief of symptoms in acute sinusitis, with a number needed to treat to benefit of seven for resolution or symptom improvement. A large primary care factorial trial is needed to establish whether oral corticosteroids offer additional benefits over antibiotics in acute sinusitis.
Conflict of interest statement
Roderick P. Venekamp and Maroeska M. Rovers were involved in the PRET (Prednisolone Rhinosinusitis Efficacy Trial) study entitled 'Optimal treatment of rhinosinusitis‐like symptoms: double‐blind placebo controlled randomised study with prednisolone versus usual care treatment' (Venekamp 2012a). This trial has been included in this 2014 update. To avoid any potential conflicts of interest, two other review authors (Gail Hayward, Paul Glasziou) performed 'Risk of bias' assessment and data extraction for this trial.
Maroeska M. Rovers has participated in a workshop and educational activities on otitis media organised by GlaxoSmithKline and received a grant from GlaxoSmithKline for a study on the microbiology of otitis media in 2009.
Paul Glasziou is an investigator on a five‐year National Health and Medical Research Council (NHMRC) grant looking at antibiotic prescribing and resistance. He is on the board of Therapeutic Guidelines Ltd, which produces the Antibiotic Guidelines booklet.
Matthew J Thompson, Gail Hayward, Carl J Heneghan, Chris B Del Mar and Rafael Perera: none known.
Figures



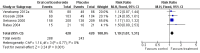













Update of
-
Systemic corticosteroids for acute sinusitis.Cochrane Database Syst Rev. 2011 Dec 7;(12):CD008115. doi: 10.1002/14651858.CD008115.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2014 Mar 25;(3):CD008115. doi: 10.1002/14651858.CD008115.pub3. PMID: 22161418 Updated.
Comment in
-
Systemic corticosteroid therapy for acute sinusitis.JAMA. 2015 Mar 24-31;313(12):1258-9. doi: 10.1001/jama.2014.14418. JAMA. 2015. PMID: 25803348
References
References to studies included in this review
Cannoni 1990 {published data only}
-
- Cannoni M, Sambuc R, San Marco JL, Auquier P, Gorget C, Chiarelli P. Comparative study of the efficacy and tolerance of prednisolone versus niflumic acid in the treatment of acute sinusitis in adults [Étude comparative de l'efficacité et de la tolérance de la prednisolone versus acide niflumide dans le traitement des sinusites aiguës de l'adulte]. Annales d Oto‐Laryngologie et de Chirurgie Cervico‐Faciale 1990;107(4):276‐81. [PUBMED: 2221720] - PubMed
Gehanno 2000 {published data only}
-
- Gehanno P, Beauvillain C, Bobin S, Chobaut JC, Desaulty A, Dubreuil C, et al. Short therapy with amoxicillin‐clavulanate and corticosteroids in acute sinusitis: results of a multicentre study in adults. Scandinavian Journal of Infectious Diseases 2000;32(6):679‐84. [PUBMED: 11200381] - PubMed
Klossek 2004 {published data only}
-
- Klossek JM, Desmonts‐Gohler C, Deslandes B, Coriat F, Bordure P, Dubreuil C, et al. Treatment of functional signs of acute maxillary rhinosinusitis in adults. Efficacy and tolerance of administration of oral prednisone for 3 days [Traitement des signes fonctionnels des rhinosinusites maxillaires aiguës de l'adulte. Efficacité et tolérance de la prednisone administrée par voie orale pendant 3 jours]. Presse Médicale 2004;33(5):303‐9. [PUBMED: 15041875] - PubMed
Ratau 2004 {published data only}
-
- Ratau NP, Snyman JR, Swanepoel C. Short‐course, low‐dose oral betamethasone as an adjunct in the treatment of acute infective sinusitis. Clinical Drug Investigation 2004;24(10):577‐82. [PUBMED: 17523719] - PubMed
References to studies excluded from this review
Ozturk 2011 {published data only}
-
- Ozturk F, Bakirtas A, Ileri F, Turktas I. Efficacy and tolerability of systemic methylprednisolone in children and adolescents with chronic rhinosinusitis: a double‐blind, placebo‐controlled randomized trial. Journal of Allergy and Clinical Immunology 2011;128(2):348‐52. [PUBMED: 21624649] - PubMed
Remer 2005 {published data only}
-
- Remer M, Polberg K, Obszanska B, Klatka J. Chronic sinusitis therapy with antibiotics (axetyl cefuroxym, clarithromycin) and steroid (prednisone). Polski Merkuriusz Lekarski 2005;19(111):343‐4. [PUBMED: 16358864] - PubMed
Vaidyanathan 2011 {published data only}
-
- Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Annals of Internal Medicine 2011;154(5):293‐302. [PUBMED: 21357906] - PubMed
Additional references
Ahovuo‐Saloranta 2011
Akkerman 2005a
Akkerman 2005b
-
- Akkerman AE, Kuyvenhoven MM, Wouden JC, Verheij TJ. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. Journal of Antimicrobial Chemotherapy 2005;56(5):930‐6. [PUBMED: 16155062] - PubMed
Ashworth 2005
Baroody 2008
-
- Baroody FM, Mucha SM, DeTineo M. Nasal challenge with allergen leads to maxillary sinus inflammation. Journal of Allergy and Clinical Immunology 2008;121(5):1126‐32. [PUBMED: 18367240] - PubMed
Berg 1986
-
- Berg O, Carenfelt C, Rystedt G, Anggård A. Occurrence of asymptomatic sinusitis in common cold and other acute ENT‐infections. Rhinology 1986;24(3):223‐5. [PUBMED: 3775189] - PubMed
Cals 2007
Damoiseaux 2008
-
- Damoiseaux RA. Antibiotics for acute rhinosinusitis. Lancet 2008;372(9633):115; author reply 116. - PubMed
Fokkens 2012
-
- Fokkens W, Lund V, Mullol J, European Position Paper on Rhinosinusitis and Nasal Polyps Group. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology Supplement 2012;3:1‐298. [PUBMED: 22764607] - PubMed
Gwaltney 1996
Hansen 1995
Hayward 2009
Hayward 2012a
Hayward 2012b
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kirtsreesakul 2004
-
- Kirtsreesakul V, Naclerio RM. Role of allergy in rhinosinusitis. Current Opinion in Allergy and Clinical Immunology 2004;4(1):17‐23. [PUBMED: 15090914] - PubMed
Kuyvenhoven 2006
-
- Kuyvenhoven M, Essen G, Schellevis F, Verheij T. Management of upper respiratory tract infections in Dutch general practice; antibiotic prescribing rates and incidences in 1987 and 2001. Family Practice 2006;23(2):175‐9. [PUBMED: 16461445] - PubMed
Laine 1998
-
- Laine K, Määttä T, Varonen H, Mäkelä M. Diagnosing acute maxillary sinusitis in primary care: a comparison of ultrasound, clinical examination and radiography. Rhinology 1998;36(1):2‐6. [PUBMED: 9569433] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell, 2011.
Lindbaek 1996
-
- Lindbaek M, Hjortdahl P, Johnson UL. Use of symptoms, signs and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Family Medicine 1996;28(3):183‐8. [PUBMED: 8900550] - PubMed
Meltzer 2004
Mygind 2001
-
- Mygind N, Nielsen LP, Hoffmann HJ, Shukla A, Blumberga G, Dahl R, et al. Mode of action of intranasal corticosteroids. Journal of Allergy and Clinical Immunology 2001;108(Suppl 1):16‐25. [PUBMED: 11449202] - PubMed
Piccirillo 2004
-
- Piccirillo J. Clinical practice: acute bacterial sinusitis. New England Journal of Medicine 2004;351(9):902‐10. [PUBMED: 15329428] - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Russell 2011
Schweiger 2010
-
- Schweiger TA, Zdanowicz M. Systemic corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. American Journal of Health‐system Pharmacy 2010;67(12):1061‐9. [PUBMED: 20554591] - PubMed
Snow 2001
-
- Snow V, Mottur‐Pilson C, Hickner JM. Principles of appropriate antibiotic use for acute sinusitis in adults. Annals of Internal Medicine 2001;134(6):495‐7. [PUBMED: 11255527] - PubMed
Van Duijn 1992
Venekamp 2012b
-
- Venekamp RP, Rovers MM, Verheij TJ, Bonten MJ, Sachs AP. Treatment of acute rhinosinusitis: discrepancy between guideline recommendations and clinical practice. Family Practice 2012;29:706‐12. [PUBMED: 22389427] - PubMed
Welschen 2004
-
- Welschen I, Kuyvenhoven M, Hoes A, Verheij T. Antibiotics for acute respiratory tract symptoms: patients' expectations, GPs' management and patient satisfaction. Family Practice 2004;21(3):234‐7. [PUBMED: 15128681] - PubMed
Williamson 2007
-
- Williamson IG, Rumsby K, Benge S, Moore M, Smith PW, Cross M, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007;298(21):2487‐96. [PUBMED: 18056902] - PubMed
Winstead 2003
-
- Winstead W. Rhinosinusitis. Primary Care 2003;30(1):137‐54. [PUBMED: 12825253] - PubMed
Young 2008
-
- Young J, Sutter A, Merenstein D, Essen GA, Kaiser L, Varonen H, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta‐analysis of individual patient data. Lancet 2008;371(9616):908‐14. [PUBMED: 18342685] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

