Comparative myogenesis in teleosts and mammals
- PMID: 24664432
- PMCID: PMC4111864
- DOI: 10.1007/s00018-014-1604-5
Comparative myogenesis in teleosts and mammals
Abstract
Skeletal myogenesis has been and is currently under extensive study in both mammals and teleosts, with the latter providing a good model for skeletal myogenesis because of their flexible and conserved genome. Parallel investigations of muscle studies using both these models have strongly accelerated the advances in the field. However, when transferring the knowledge from one model to the other, it is important to take into account both their similarities and differences. The main difficulties in comparing mammals and teleosts arise from their different temporal development. Conserved aspects can be seen for muscle developmental origin and segmentation, and for the presence of multiple myogenic waves. Among the divergences, many fish have an indeterminate growth capacity throughout their entire life span, which is absent in mammals, thus implying different post-natal growth mechanisms. This review covers the current state of the art on myogenesis, with a focus on the most conserved and divergent aspects between mammals and teleosts.
Figures




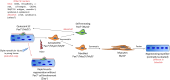
References
-
- Wilting J, Brand-Saberi B, Huang R, Zhi Q, Kontges G, Ordahl CP, Christ B. Angiogenic potential of the avian somite. Dev Dyn. 1995;202(2):165–171. - PubMed
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–695. - PubMed
-
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol. 1995;191(5):381–396. - PubMed
-
- Cinnamon Y, Kahane N, Bachelet I, Kalcheim C. The sub-lip domain–a distinct pathway for myotome precursors that demonstrate rostral-caudal migration. Development. 2001;28(3):341–351. - PubMed
-
- Rescan PY. Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp Biochem Physiol B. 2001;130(1):1–12. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

