Modulation of vacuolar pH is required for replication of Edwardsiella ictaluri in channel catfish macrophages
- PMID: 24664505
- PMCID: PMC4019175
- DOI: 10.1128/IAI.01616-13
Modulation of vacuolar pH is required for replication of Edwardsiella ictaluri in channel catfish macrophages
Abstract
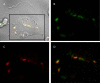
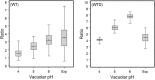
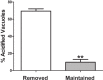
Previous in vitro work demonstrated that Edwardsiella ictaluri produces an acid-activated urease that can modulate environmental pH through the production of ammonia from urea. Additional work revealed that expression of the E. ictaluri type III secretion system (T3SS) is upregulated by acidic pH. Both the urease and the T3SS were previously shown to be essential to intracellular replication. In this work, fluorescence microscopy with LysoTracker Red DND-99 (LTR) indicated that E. ictaluri-containing vacuoles (ECV) became acidified following ingestion by head kidney-derived macrophages (HKDM). In vivo ratiometric imaging demonstrated a lowered ECV pH, which fell to as low as pH 4 but subsequently increased to pH 6 or greater. Inhibition of vacuolar H(+)-ATPases by use of the specific inhibitor bafilomycin A1 abrogated both ECV acidification and intracellular replication in HKDM. Failure of an E. ictaluri urease knockout mutant to increase the ECV pH in the in vivo ratiometric assay suggests that ammonia produced by the urease reaction mediates the pH increase. Additionally, when the specific arginase inhibitor l-norvaline was used to treat E. ictaluri-infected HKDM, the ECV failed to neutralize and E. ictaluri was unable to replicate. This indicates that the HKDM-encoded arginase enzyme produces the urea used by the E. ictaluri urease enzyme. Failure of the ECV to acidify would prevent both upregulation of the T3SS and activation of the urease enzyme, either of which would prevent E. ictaluri from replicating in HKDM. Failure of the ECV to neutralize would result in a vacuolar pH too low to support E. ictaluri replication.
Figures








References
-
- Hawke JP. 1979. A bacterium associated with disease of pond cultured catfish, Ictalurus punctatus. J. Fish. Res. Board Can. 36:1508–1512. 10.1139/f79-219 - DOI
-
- USDA. 2010. Catfish 2010 part I: reference of catfish health and production practices in the United States, 2009. USDA-APHIS-VS, CEAH, Ft. Collins, CO
-
- Thune RL, Stanley LA, Cooper RK. 1993. Bacterial diseases of catfishes, p 511–520 In Stoskopf MK. (ed), Fish medicine. W B Saunders Company, Philadelphia, PA
-
- Booth NJ, El Kamel A, Thune RL. 2006. Intracellular replication of Edwardsiella ictaluri in channel catfish macrophages. J. Aquat. Anim. Health 18:101–108. 10.1577/H05-025.1 - DOI
-
- Thune RL, Fernandez DH, Benoit JL, Kelly-Smith M, Rogge ML, Booth NJ, Landry CA, Bologna RA. 2007. Signature-tagged mutagenesis of Edwardsiella ictaluri identifies virulence-related genes, including a salmonella pathogenicity island 2 class of type III secretion systems. Appl. Environ. Microbiol. 73:7934–7946. 10.1128/AEM.01115-07 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

