A randomized clinical trial comparing the efficacy and safety of ramosetron versus ondansetron in patients undergoing abdominal surgery under general anesthesia
- PMID: 24665241
- PMCID: PMC3950454
- DOI: 10.4103/1658-354X.125938
A randomized clinical trial comparing the efficacy and safety of ramosetron versus ondansetron in patients undergoing abdominal surgery under general anesthesia
Abstract
Background: Post-operative nausea and vomiting is one of the most common and distressing complications after anesthesia and surgery. It may lead to serious post-operative complications. Ramosetron is a newer 5-HT3 receptor antagonist and has more potent and longer duration of antiemetic effects compared to first generation 5HT3 receptor antagonists. The purpose of this study was to compare the efficacy of Ramosetron for the prevention of post-operative nausea and vomiting with that of Ondansetron in patients undergoing abdominal surgeries under general anesthesia.
Methods: In this randomized, double-blind study, 60 patients, 18-60 years of both genders falling under ASA I-II category scheduled for abdominal surgery were included. Group I received I.V ramosetron 0.3 mg while group II received I.V Ondansetron 4 mg at the time of extubation. The standard general anesthetic technique was used throughout. Postoperatively the incidences of nausea, vomiting, and safety assessments were performed at 1, 2, 6, and 24 h during the first 24 h after surgery.
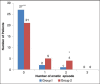
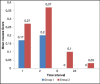
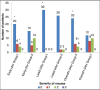
Results: There were no differences between groups with respect to patient demographics. The percentage of patients who had complete response (no PONV, and no need for another rescue antiemetic) from 0 to 24 h after anesthesia was 56% with ramosetron and 33% with ondansetron. The corresponding rates at 1, 2, 6, and 24 h after anesthesia were 76% and 63%, 76% and 50%, 100 and 83%, 100 and 93%, respectively. Safety profiles of the two drugs were comparable, as no clinically serious adverse effects caused by study drugs were observed in either of the groups.
Conclusion: Our study concludes that prophylactic therapy with ramosetron is highly efficacious than ondansetron in preventing PONV in patients undergoing abdominal surgery under general anesthesia.
Keywords: General anesthesia; ondansetron; post-operative vomiting; postoperative nausea; ramosetron.
Conflict of interest statement
Figures
References
-
- Kapur PA. The big “Little problem”. Anesth Analg. 1991;73:243–5. - PubMed
-
- Williams KS. Postoperative nausea and vomiting. Surg Clin North Am. 2005;85:1229–41. - PubMed
-
- Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43. - PubMed
-
- Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for ambulatory anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. - PubMed
-
- Watcha MF, White PF. Postoperative nausea and vomiting: Do they matter? Eur J Anaesthesiology. 1995;12(Suppl 10):18–23. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources