Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells
- PMID: 24665390
- PMCID: PMC3875607
- DOI: 10.4161/tisb.24333
Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells
Abstract
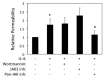
Emerging evidence indicates that airway epithelial barrier function is compromised in asthma, a disease characterized by Th2-skewed immune response against inhaled allergens, but the mechanisms involved are not well understood. The purpose of this study was to investigate the effects of Th2-type cytokines on airway epithelial barrier function. 16HBE14o- human bronchial epithelial cells monolayers were grown on collagen coated Transwell inserts. The basolateral or apical surfaces of airway epithelia were exposed to human interleukin-4 (IL-4), IL-13, IL-25, IL-33, thymic stromal lymphopoietin (TSLP) alone or in combination at various concentrations and time points. We analyzed epithelial apical junctional complex (AJC) function by measuring transepithelial electrical resistance (TEER) and permeability to FITC-conjugated dextran over time. We analyzed AJC structure using immunofluorescence with antibodies directed against key junctional components including occludin, ZO-1, β-catenin and E-cadherin. Transepithelial resistance was significantly decreased after both basolateral and apical exposure to IL-4. Permeability to 3 kDa dextran was also increased in IL-4-exposed cells. Similar results were obtained with IL-13, but none of the innate type 2 cytokines examined (TSLP, IL-25 or IL-33) significantly affected barrier function. IL-4 and IL-13-induced barrier dysfunction was accompanied by reduced expression of membrane AJC components but not by induction of claudin- 2. Enhanced permeability caused by IL-4 was not affected by wortmannin, an inhibitor of PI3 kinase signaling, but was attenuated by a broad spectrum inhibitor of janus associated kinases. Our study indicates that IL-4 and IL-13 have disruptive effect on airway epithelial barrier function. Th2-cytokine induced epithelial barrier dysfunction may contribute to airway inflammation in allergic asthma.
Keywords: IL-13; IL-25; IL-33; IL-4; Janus associated kinase (JAK); TSLP; Th2 cytokines; adherens junction; airway epithelial cell; allergy; apical junctional complex; asthma; barrier dysfunction; tight junction.
Figures




References
-
- Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, et al. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol. 2011;128:1216–24, e11. doi: 10.1016/j.jaci.2011.08.035. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
