Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment
- PMID: 24666324
- PMCID: PMC4243911
- DOI: 10.1111/bcp.12362
Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment
Abstract
Aims: In September 2012 the UK's Commission on Human Medicines (CHM) recommended changes in the management of paracetamol poisoning: use of a single '100 mg l(-1) ' nomogram treatment line, ceasing risk assessment, treating all staggered/uncertain ingestions and increasing the duration of the initial acetylcysteine (NAC) infusion from 15 to 60 min. We evaluated the effect of this on presentation, admission, treatment, adverse reactions and costs of paracetamol poisoning.
Methods: Data were prospectively collected from adult patients presenting to three large UK hospitals from 3 September 2011 to 3 September 2013 (year before and after change). Infusion duration effect on vomiting and anaphylactoid reactions was examined in one centre. A cost analysis from an NHS perspective was performed for 90 000 patients/annum with paracetamol overdose.
Results: There were increases in the numbers presenting to hospital (before 1703, after 1854; increase 8.9% [95% CI 1.9, 16.2], P = 0.011); admitted (1060/1703 [62.2%] vs. 1285/1854 [69.3%]; increase 7.1% [4.0, 10.2], P < 0.001) and proportion treated (626/1703 [36.8%] vs. 926/1854 [50.0%]; increase: 13.2% [95% CI 10.0, 16.4], P < 0.001). Increasing initial NAC infusion did not change the proportion of treated patients developing adverse reactions (15 min 87/323 [26.9%], 60 min 145/514 [28.2%]; increase: 1.3% [95% CI -4.9, 7.5], P = 0.682). Across the UK the estimated cost impact is £8.3 million (6.4 million-10.2 million) annually, with a cost-per-life saved of £17.4 million (13.4 million-21.5 million).
Conclusions: The changes introduced by the CHM in September 2012 have increased the numbers of patients admitted to hospital and treated with acetylcysteine without reducing adverse reactions. A safety and cost-benefit review of the CHM guidance is warranted, including novel treatment protocols and biomarkers in the assessment of poisoning.
Keywords: acetylcysteine; adverse effects; paracetamol; poisoning; regulation.
© 2014 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
References
-
- Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. - PubMed
-
- McQuade DJ, Dargan PI, Keep J, Wood MD. Paracetamol toxicity: what would be the implications of a change in UK treatment guidelines? Eur J Clin Pharmacol. 2012;65:1541–1547. - PubMed
-
- MHRA. 2012. Benefit risk profile of acetylcysteine in the management of paracetamol overdose. [Internet]. Available at: http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymessage/con1... (last accessed 13 May 2013)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

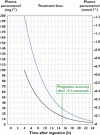
 , normal treatment…
, normal treatment…  , high risk treatment line
, high risk treatment line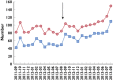
 ) and…
) and…  ) for paracetamol poisoning with acetylcysteine…
) for paracetamol poisoning with acetylcysteine… 