Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies
- PMID: 24666336
- PMCID: PMC4008980
- DOI: 10.1111/cei.12301
Neutrophils from vasculitis patients exhibit an increased propensity for activation by anti-neutrophil cytoplasmic antibodies
Abstract
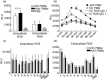
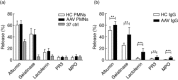
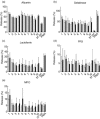
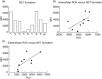
Anti-neutrophil cytoplasmic antibodies (ANCA) are thought to be pathogenic in ANCA-associated vasculitis (AAV) by stimulating polymorphonuclear leucocytes (PMNs) to degranulate and produce reactive oxygen species (ROS). The aim of this study was to investigate if PMNs from AAV patients are stimulated more readily by ANCA compared with PMNs from healthy controls (HCs). Differences in ANCA characteristics that can account for different stimulation potential were also studied. PMNs from five AAV patients and five HCs were stimulated with 10 different immunoglobulins (Ig)Gs, purified from PR3-ANCA-positive patients, and ROS production, degranulation and neutrophil extracellular trap (NET) formation was measured. ANCA levels, affinity and clinical data of the AAV donors were recorded. The results show that PMNs from AAV patients produce more intracellular ROS (P = 0·019), but degranulate to a similar extent as PMNs from HCs. ROS production correlated with NET formation. Factors that may influence the ability of ANCA to activate PMNs include affinity and specificity for N-terminal epitopes. In conclusion, our results indicate that PMNs from AAV patients in remission behave quite similarly to HC PMNs, with the exception of a greater intracellular ROS production. This could contribute to more extensive NET formation and thus an increased exposure of the ANCA autoantigens to the immune system.
Keywords: ANCA-associated vasculitis; autoimmunity; degranulation; reactive oxygen species.
© 2014 British Society for Immunology.
Figures

References
-
- Yang JJ, Preston GA, Alcorta DA, et al. Expression profile of leukocyte genes activated by anti-neutrophil cytoplasmic autoantibodies (ANCA) Kidney Int. 2002;62:1638–1649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

