The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice
- PMID: 24666588
- PMCID: PMC4233996
- DOI: 10.1111/cea.12312
The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice
Abstract
Background: Allergen-specific immunotherapy favours immune deviation from a Th2 to a Th1 response and increases the number of regulatory T cells (Tregs). Epicutaneous immunotherapy (EPIT) of sensitized mice decreases the clinical and the allergen-specific Th2 responses and increases local and peripheral Foxp3(+) Tregs.
Objective: To investigate the role of Tregs in EPIT and characterize their phenotype and maintenance following EPIT.
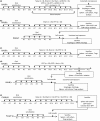
Methods: Tregs were investigated using in vivo depletion or adoptive transfer into BALB/c mice. Tregs were depleted using anti-CD25 antibody injection during EPIT, and allergen-specific responses were compared with Sham, EPIT alone and naïve mice. To demonstrate that Tregs can mediate protection by their own, and to study their maintenance following the end of EPIT, CD25(+) CD4(+) Tregs isolated from mice just after or 8 weeks after EPIT were transferred into peanut-sensitized mice. Foxp3-IRES-mRFP mice were transferred with EPIT-induced Tregs to analyse the induction of host Tregs.
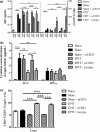
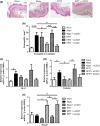
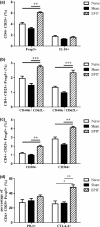
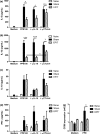
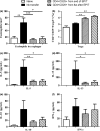
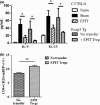
Results: The anti-CD25 antibody injection to EPIT mice abrogated the induction of Tregs in spleen and the expression of Foxp3 in oesophagus. This resulted in levels of peanut-induced eosinophilic infiltration in oesophagus similar to Sham and significantly higher than EPIT. Whereas the transfer of Tregs from Sham-treated mice demonstrated no effect, the transfer of Tregs isolated just after EPIT prevented peanut-induced eosinophil infiltration and eotaxin expression and induced Foxp3 in oesophagus. The transfer of Tregs isolated 8 weeks after EPIT suppressed allergen-specific responses as efficiently as did Tregs isolated just after EPIT and increased spleen Foxp3(+) CD25(+) CD4(+) cells similarly. The use of reporter mice demonstrated an increase in host Tregs.
Conclusions: These results confirm the Tregs-mediated mechanism of EPIT and demonstrate the persistence of efficient Tregs during a long period of time after treatment cessation. This suggests that EPIT induces long-term tolerance in peanut-sensitized mice.
Keywords: epicutaneous immunotherapy; peanut allergy; regulatory T cells.
© 2014 DBV Technologies. Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut.Cell Mol Immunol. 2017 Sep;14(9):770-782. doi: 10.1038/cmi.2016.14. Epub 2016 Apr 11. Cell Mol Immunol. 2017. PMID: 27063469 Free PMC article.
-
Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model.J Allergy Clin Immunol. 2015 Jun;135(6):1546-57.e4. doi: 10.1016/j.jaci.2014.11.028. Epub 2015 Jan 9. J Allergy Clin Immunol. 2015. PMID: 25583102
-
CD4(+) CD25(+) T regulatory cells do not transfer oral tolerance to peanut allergens in a mouse model of peanut allergy.Clin Exp Allergy. 2011 Sep;41(9):1324-33. doi: 10.1111/j.1365-2222.2010.03662.x. Epub 2011 Feb 21. Clin Exp Allergy. 2011. PMID: 21338425
-
Epicutaneous immunotherapy for the treatment of peanut allergy.Allergy. 2025 Jan;80(1):63-76. doi: 10.1111/all.16324. Epub 2024 Sep 28. Allergy. 2025. PMID: 39340442 Free PMC article. Review.
-
Immunotherapy approaches for peanut allergy.Expert Rev Clin Immunol. 2020 Feb;16(2):167-174. doi: 10.1080/1744666X.2019.1708192. Epub 2020 Jan 12. Expert Rev Clin Immunol. 2020. PMID: 31928251 Review.
Cited by
-
An Examination of Clinical and Immunologic Outcomes in Food Allergen Immunotherapy by Route of Administration.Curr Allergy Asthma Rep. 2015 Jun;15(6):35. doi: 10.1007/s11882-015-0536-y. Curr Allergy Asthma Rep. 2015. PMID: 26141581 Review.
-
Preclinical evaluation of alternatives to oral immunotherapy for food allergies.Front Allergy. 2023 Oct 3;4:1275373. doi: 10.3389/falgy.2023.1275373. eCollection 2023. Front Allergy. 2023. PMID: 37859977 Free PMC article. Review.
-
Improving the safety of oral immunotherapy for food allergy.Pediatr Allergy Immunol. 2016 Mar;27(2):117-25. doi: 10.1111/pai.12510. Epub 2015 Dec 22. Pediatr Allergy Immunol. 2016. PMID: 26593873 Free PMC article. Review.
-
Innate and adaptive dendritic cell responses to immunotherapy.Curr Opin Allergy Clin Immunol. 2015 Dec;15(6):575-80. doi: 10.1097/ACI.0000000000000213. Curr Opin Allergy Clin Immunol. 2015. PMID: 26509662 Free PMC article. Review.
-
Desensitization for Peanut Allergies in Children.Curr Treat Options Allergy. 2016 Sep;3(3):282-291. doi: 10.1007/s40521-016-0091-3. Epub 2016 Jul 21. Curr Treat Options Allergy. 2016. PMID: 28042528 Free PMC article.
References
-
- Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–46. - PubMed
-
- Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–15. - PubMed
-
- O'Brien RM, Byron KA, Varigos GA, Thomas WR. House dust mite immunotherapy results in a decrease in Der p 2-specific IFN-gamma and IL-4 expression by circulating T lymphocytes. Clin Exp Allergy. 1997;27:46–51. - PubMed
-
- Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–13. - PubMed
-
- Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

