Ruscogenin ameliorates diabetic nephropathy by its anti-inflammatory and anti-fibrotic effects in streptozotocin-induced diabetic rat
- PMID: 24666993
- PMCID: PMC3986976
- DOI: 10.1186/1472-6882-14-110
Ruscogenin ameliorates diabetic nephropathy by its anti-inflammatory and anti-fibrotic effects in streptozotocin-induced diabetic rat
Abstract
Background: Ruscogenin is a major steroid sapogenin in the traditional Chinese herb Ophiopogon japonicus that have multiple bioactivities. Recent studies have demonstrated that ruscogenin is involved in down-regulation of intercellular adhesion molecule-1 (ICAM-1) and nuclear factor-κB (NF-κB) activation in anti-inflammatory pathways. We hypothesized that ruscogenin protects against diabetic nephropathy (DN) by inhibiting NF-κB-mediated inflammatory pathway. To test this hypothesis, the present study was to examine the effects of ruscogenin in rats with streptozotocin (STZ)-induced DN.
Methods: Diabetes was induced with STZ (60 mg/kg) by intraperitoneal injection in rats. Two weeks after STZ injection, rats in the treatment group were orally dosed with 0.3, 1.0 or 3.0 mg/kg ruscogenin for 8 weeks. The normal rats were chosen as nondiabetic control group. The rats were sacrificed 10 weeks after induction of diabetes. Changes in renal function-related parameters in plasma and urine were analyzed at the end of the study. Kidneys were isolated for pathology histology, immunohistochemistry, and Western blot analyses.
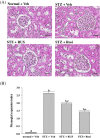
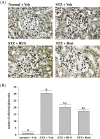
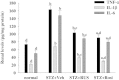
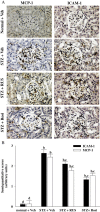
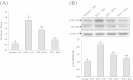
Results: Ruscogenin administration did not lower the levels of plasma glucose and glycosylated hemoglobin in STZ-diabetic rats. Diabetic rats exhibited renal dysfunction, as evidenced by reduced creatinine clearance, blood urea nitrogen and proteinuria, along with marked elevation in the ratio of kidney weight to body weight, that were reversed by ruscogenin. Ruscogenin treatment was found to markedly improve histological architecture in the diabetic kidney. Renal NF-κB activity, as wells as protein expression and infiltration of macrophages were increased in diabetic kidneys, accompanied by an increase in protein content of intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in kidney tissues. All of the above abnormalities were reversed by ruscogenin treatment, which also decreased the expression of transforming growth factor-β1 and fibronectin in the diabetic kidneys.
Conclusions: Our data demonstrated that ruscogenin suppressed the inflammation and ameliorated the structural and functional abnormalities of the diabetic kidney in rats might be associated with inhibition of NF-κB mediated inflammatory genes expression.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

