Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses
- PMID: 24667168
- PMCID: PMC3965421
- DOI: 10.1371/journal.pone.0092153
Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses
Abstract
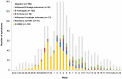
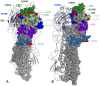
Background: Influenza vaccine effectiveness (VE) is generally interpreted in the context of vaccine match/mismatch to circulating strains with evolutionary drift in the latter invoked to explain reduced protection. During the 2012-13 season, however, detailed genotypic and phenotypic characterization shows that low VE was instead related to mutations in the egg-adapted H3N2 vaccine strain rather than antigenic drift in circulating viruses.
Methods/findings: Component-specific VE against medically-attended, PCR-confirmed influenza was estimated in Canada by test-negative case-control design. Influenza A viruses were characterized genotypically by amino acid (AA) sequencing of established haemagglutinin (HA) antigenic sites and phenotypically through haemagglutination inhibition (HI) assay. H3N2 viruses were characterized in relation to the WHO-recommended, cell-passaged vaccine prototype (A/Victoria/361/2011) as well as the egg-adapted strain as per actually used in vaccine production. Among the total of 1501 participants, influenza virus was detected in 652 (43%). Nearly two-thirds of viruses typed/subtyped were A(H3N2) (394/626; 63%); the remainder were A(H1N1)pdm09 (79/626; 13%), B/Yamagata (98/626; 16%) or B/Victoria (54/626; 9%). Suboptimal VE of 50% (95%CI: 33-63%) overall was driven by predominant H3N2 activity for which VE was 41% (95%CI: 17-59%). All H3N2 field isolates were HI-characterized as well-matched to the WHO-recommended A/Victoria/361/2011 prototype whereas all but one were antigenically distinct from the egg-adapted strain as per actually used in vaccine production. The egg-adapted strain was itself antigenically distinct from the WHO-recommended prototype, and bore three AA mutations at antigenic sites B [H156Q, G186V] and D [S219Y]. Conversely, circulating viruses were identical to the WHO-recommended prototype at these positions with other genetic variation that did not affect antigenicity. VE was 59% (95%CI:16-80%) against A(H1N1)pdm09, 67% (95%CI: 30-85%) against B/Yamagata (vaccine-lineage) and 75% (95%CI: 29-91%) against B/Victoria (non-vaccine-lineage) viruses.
Conclusions: These findings underscore the need to monitor vaccine viruses as well as circulating strains to explain vaccine performance. Evolutionary drift in circulating viruses cannot be regulated, but influential mutations introduced as part of egg-based vaccine production may be amenable to improvements.
Conflict of interest statement
Figures

References
-
- Skowronski DM, Janjua NZ, De Serres G, Dickinson JA, Winter AL, et al. (2013) Interim estimates of influenza vaccine effectiveness in 2012/13 from Canada's sentinel surveillance network, January 2013. Euro Surveill 18 ((5)) 7–16 Available: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20394. Accessed 2013 Dec 7. - PubMed
-
- Public Health Agency of Canada. FluWatch. Available: http://www.phac-aspc.gc.ca/fluwatch/12-13/index-eng.php. Accessed 2013 Dec 7.
-
- National Advisory Committee on Immunization (NACI) (2013) Statement on seasonal influenza vaccine for 2013–2014. Can Commun Dis Rep 39: 1–37 Available: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/13vol39/acs-dcc-4/index-en.... Accessed 2013 Dec 7. - PMC - PubMed
-
- Bresee J, Hayden FG (2013) Epidemic influenza—responding to the expected but unpredictable. N Engl J Med 368: 589–592 published online February 14. Available: http://www.nejm.org/doi/full/10.1056/NEJMp1300375. Accessed 2013 Dec 7. - DOI - PubMed
-
- Centers for Disease Control and Prevention (CDC). FluView. Atlanta, GA: CDC. Available: http://www.cdc.gov/flu/weekly/pastreports.htm. Accessed 2013 Dec 7.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

