The role of macrophage migration inhibitory factor in anesthetic-induced myocardial preconditioning
- PMID: 24667295
- PMCID: PMC3965449
- DOI: 10.1371/journal.pone.0092827
The role of macrophage migration inhibitory factor in anesthetic-induced myocardial preconditioning
Abstract
Introduction: Anesthetic-induced preconditioning (AIP) is known to elicit cardioprotective effects that are mediated at least in part by activation of the kinases AMPK and PKCε as well as by inhibition of JNK. Recent data demonstrated that the pleiotropic cytokine macrophage migration inhibitory factor (MIF) provides cardioprotection through activation and/or inhibition of kinases that are also known to mediate effects of AIP. Therefore, we hypothesized that MIF could play a key role in the AIP response.
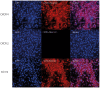
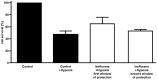
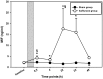
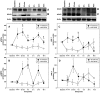
Methods: Cardiomyocytes were isolated from rats and subjected to isoflurane preconditioning (4 h; 1.5 vol. %). Subsequently, MIF secretion and alterations in the activation levels of protective kinases were compared to a control group that was exposed to ambient air conditions. MIF secretion was quantified by ELISA and AIP-induced activation of protein kinases was assessed by Western blotting of cardiomyocyte lysates after isoflurane treatment.
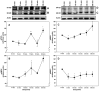
Results: In cardiomyocytes, preconditioning with isoflurane resulted in a significantly elevated secretion of MIF that followed a biphasic behavior (30 min vs. baseline: p = 0.020; 24 h vs. baseline p = 0.000). Moreover, quantitative polymerase chain reaction demonstrated a significant increase in MIF mRNA expression 8 h after AIP. Of note, activation of AMPK and PKCε coincided with the observed peaks in MIF secretion and differed significantly from baseline.
Conclusions: These results suggest that the pleiotropic mediator MIF is involved in anesthetic-induced preconditioning of cardiomyocytes through stimulation of the protective kinases AMPK and PKCε.
Conflict of interest statement
Figures


References
-
- Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136. - PubMed
-
- Reimer KA, Murry CE, Jennings RB (1990) Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation 82: 2266–2268. - PubMed
-
- Weber NC, Schlack W (2008) Inhalational anaesthetics and cardioprotection. Handb Exp Pharmacol 182: 187–207. - PubMed
-
- Pagel PS (2010) Cardioprotection by noble gases. J Cardiothorac Vasc Anesth 24: 143–163. - PubMed
-
- Frässdorf J, De Hert S, Schlack W (2009) Anaesthesia and myocardial ischaemia/reperfusion injury. Br J of Anaesth 103: 89–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

