Effects of high affinity leptin antagonist on prolactin receptor deficient male mouse
- PMID: 24667351
- PMCID: PMC3965386
- DOI: 10.1371/journal.pone.0091422
Effects of high affinity leptin antagonist on prolactin receptor deficient male mouse
Abstract
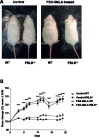
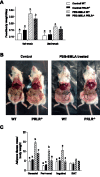
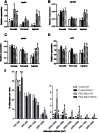
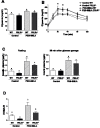
Hyperprolactinemia occurs during gestation and lactation with marked hyperphagia associated with leptin resistance. Prolactin (PRL) induces the expression of orexigenic neuropeptide Y (NPY) in hypothalamic dorsomedial nucleus (DMH) leading to hyperphagia. Along this line prolactin receptor deficient (PRLR-/-) mice are resistant to obesity under high fat diet due to increased energy expenditure. As these mice have an altered food intake, our objective was to test whether leptin is responsible for these characteristics. PRLR-/- male mice and control littermates were injected subcutaneously every other day with 12 mg/kg pegylated superactive mouse leptin antagonist (PEG-SMLA) for 3 weeks. We tested the effect of PEG-SMLA on body weight, food intake and metabolic parameters. The antagonist led to a rapid increase in body weight (20%) but increased adipose mass in PEG-SMLA treated mice was less pronounced in PRLR-/- than in WT mice. Food intake of PEG-SMLA-injected animals increased during the first week period of the experiment but then declined to a similar level of the control animals during the second week. Interestingly, PRLR-/- mice were found to have the same bone volume than those of control mice although PEG-SMLA increased bone mass by 7% in both strains. In addition, PEG-SMLA led to insulin resistance and glucose intolerance as well as an altered lipid profile in treated mice. Altogether, these results suggest that PRLR-/- mice respond to leptin antagonist similarly to the control mice, indicating no interaction between the actions of the two hormones.
Conflict of interest statement
Figures
References
-
- Sauvé D, Woodside B (2000) Neuroanatomical specificity of prolactin-induced hyperphagia in virgin female rats. Brain Res 868: 306–314. - PubMed
-
- Ling C, Hellgren G, Gebre-Medhin M, Dillner K, Wennbo H, et al. (2000) Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology 141: 3564–3572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

