p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium
- PMID: 24667411
- PMCID: PMC4019388
- DOI: 10.1016/j.bbamcr.2014.03.016
p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium
Abstract
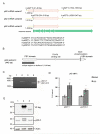
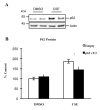
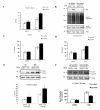
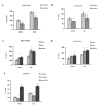
As a signaling hub, p62/sequestosome plays important roles in cell signaling and degradation of misfolded proteins. p62 has been implicated as an adaptor protein to mediate autophagic clearance of insoluble protein aggregates in age-related diseases, including age-related macular degeneration (AMD), which is characterized by dysfunction of the retinal pigment epithelium (RPE). Our previous studies have shown that cigarette smoke (CS) induces oxidative stress and inhibits the proteasome pathway in cultured human RPE cells, suggesting that p62-mediated autophagy may become the major route to remove impaired proteins under such circumstances. In the present studies, we found that all p62 mRNA variants are abundantly expressed and upregulated by CS induced stress in cultured human RPE cells, yet isoform1 is the major translated form. We also show that p62 silencing exacerbated the CS induced accumulation of damaged proteins, both by suppressing autophagy and by inhibiting the Nrf2 antioxidant response, which in turn, increased protein oxidation. These effects of CS and p62 reduction were further confirmed in mice exposed to CS. We found that over-expression of p62 isoform1, but not its S403A mutant, which lacks affinity for ubiquitinated proteins, reduced misfolded proteins, yet simultaneously promoted an Nrf2-mediated antioxidant response. Thus, p62 provides dual, reciprocal enhancing protection to RPE cells from environmental stress induced protein misfolding and aggregation, by facilitating autophagy and the Nrf2 mediated antioxidant response, which might be a potential therapeutic target against AMD.
Keywords: Aging; Autophagy; Nrf2; Oxidative stress; p62.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells.Autophagy. 2015;11(9):1636-51. doi: 10.1080/15548627.2015.1061170. Autophagy. 2015. PMID: 26237736 Free PMC article.
-
Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells.Mol Vis. 2014 Jun 2;20:760-9. eCollection 2014. Mol Vis. 2014. PMID: 24940030 Free PMC article.
-
Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells.PLoS One. 2013 Jul 29;8(7):e69563. doi: 10.1371/journal.pone.0069563. Print 2013. PLoS One. 2013. PMID: 23922739 Free PMC article.
-
Biology of p62/sequestosome-1 in Age-Related Macular Degeneration (AMD).Adv Exp Med Biol. 2016;854:17-22. doi: 10.1007/978-3-319-17121-0_3. Adv Exp Med Biol. 2016. PMID: 26427388 Review.
-
Nrf2 signaling is impaired in the aging RPE given an oxidative insult.Exp Eye Res. 2014 Feb;119:111-4. doi: 10.1016/j.exer.2013.10.024. Epub 2013 Nov 8. Exp Eye Res. 2014. PMID: 24216314 Free PMC article. Review.
Cited by
-
Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress.Oxid Med Cell Longev. 2020 Aug 8;2020:2896036. doi: 10.1155/2020/2896036. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32831993 Free PMC article. Review.
-
Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration.JCI Insight. 2021 May 10;6(9):e142254. doi: 10.1172/jci.insight.142254. JCI Insight. 2021. PMID: 33822768 Free PMC article.
-
Microtubule-Associated Protein 1 Light Chain 3B, (LC3B) Is Necessary to Maintain Lipid-Mediated Homeostasis in the Retinal Pigment Epithelium.Front Cell Neurosci. 2018 Oct 8;12:351. doi: 10.3389/fncel.2018.00351. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30349463 Free PMC article.
-
Mitochondrial stress triggers a pro-survival response through epigenetic modifications of nuclear DNA.Cell Mol Life Sci. 2019 Apr;76(7):1397-1417. doi: 10.1007/s00018-019-03008-5. Epub 2019 Jan 23. Cell Mol Life Sci. 2019. PMID: 30673822 Free PMC article.
-
MicroRNA-100 Mediates Hydrogen Peroxide-Induced Apoptosis of Human Retinal Pigment Epithelium ARPE-19 Cells.Pharmaceuticals (Basel). 2021 Apr 1;14(4):314. doi: 10.3390/ph14040314. Pharmaceuticals (Basel). 2021. PMID: 33915898 Free PMC article.
References
-
- Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Molecular cell. 2011;44:279–289. - PubMed
-
- Babu JR, Geetha T, Wooten MW. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. Journal of neurochemistry. 2005;94:192–203. - PubMed
-
- Braak H, Thal DR, Del Tredici K. Nerve cells immunoreactive for p62 in select hypothalamic and brainstem nuclei of controls and Parkinson’s disease cases. J Neural Transm. 2011;118:809–819. - PubMed
-
- Rue L, Lopez-Soop G, Gelpi E, Martinez-Vicente M, Alberch J, Perez-Navarro E. Brain region- and age-dependent dysregulation of p62 and NBR1 in a mouse model of Huntington’s disease. Neurobiology of disease. 2013;52:219–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

