Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis
- PMID: 24667579
- PMCID: PMC3965555
- DOI: 10.1371/journal.pone.0093293
Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis
Abstract
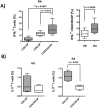
Peripheral CD4CD8 double positive (DP) T cells have been reported to play a role in several autoimmune diseases, virus infections and cancer. In rheumatoid arthritis (RA), both CD4 and CD8 single positive (SP) T cells are known to be involved in the pathogenesis, but the role of peripheral CD4CD8 DP T cells has not been investigated in detail. Anti cyclic citrullinated antibodies (ACPA) positive and ACPA negative RA patients, patients with systemic lupus erythematodes (SLE) and age matched healthy donors (HD) were enrolled in the analysis. The frequencies and phenotype of DP T cells in PBMC were investigated. In addition, DP T cells were quantified in biopsies from rheumatoid synovium. After in vitro restimulation, the cytokine production of DP T cells was investigated in cultures of PBMC. CMV specific cytokine secretion as well as proliferation was analyzed following antigen specific restimulation after an appropriate culture duration. DP T cells were found more frequently in RA patients than in healthy controls or patients with SLE. These DP T cells express αβ TCRs, are of a memory phenotype and share features of both CD4 as well as CD8 SP T cells. Importantly, DP T cells were found to also be present in the rheumatoid synovium. Further characterization of DP T cells from RA patients revealed increased production of IL-21 and IL-4, implying a possible role as T helper cells. In addition, DP T cells in RA seem to contribute to the inflammatory process, because they produce significantly more IFNγ than counterparts from HD and are increased in CMV+ RA patients. Given their capacity to produce a variety of cytokines (IL4, IL21 and IFNγ), their association with ACPA positive RA and their presence in the synovium, we suggest an important role of double positive T cells in the pathogenesis of rheumatoid arthritis.
Conflict of interest statement
Figures




References
-
- Pierer M, Rothe K, Quandt D, Schulz A, Rossol M, et al. (2012) Association of anticytomegalovirus seropositivity with more severe joint destruction and more frequent joint surgery in rheumatoid arthritis. Arthritis Rheum 64(6): 1740–1749. - PubMed
-
- Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B, et al. (2004) Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 104(2): 478–486. - PubMed
-
- Parel Y, Chizzolini C (2004) CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev 3(3): 215–220. - PubMed
-
- Zloza A, Al-Harthi L (2006) Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J Leukoc Biol 79(1): 4–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

