Cloning and characterization of a wheat homologue of apurinic/apyrimidinic endonuclease Ape1L
- PMID: 24667595
- PMCID: PMC3965494
- DOI: 10.1371/journal.pone.0092963
Cloning and characterization of a wheat homologue of apurinic/apyrimidinic endonuclease Ape1L
Erratum in
- PLoS One. 2014;9(6):e101795
Abstract
Background: Apurinic/apyrimidinic (AP) endonucleases are key DNA repair enzymes involved in the base excision repair (BER) pathway. In BER, an AP endonuclease cleaves DNA at AP sites and 3'-blocking moieties generated by DNA glycosylases and/or oxidative damage. A Triticum aestivum cDNA encoding for a putative homologue of ExoIII family AP endonucleases which includes E. coli Xth, human APE1 and Arabidopsis thaliana AtApe1L has been isolated and its protein product purified and characterized.
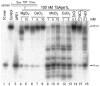
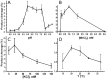
Methodology/principal findings: We report that the putative wheat AP endonuclease, referred here as TaApe1L, contains AP endonuclease, 3'-repair phosphodiesterase, 3'-phosphatase and 3' → 5' exonuclease activities. Surprisingly, in contrast to bacterial and human AP endonucleases, addition of Mg(2+) and Ca(2+) (5-10 mM) to the reaction mixture inhibited TaApe1L whereas the presence of Mn(2+), Co(2+) and Fe(2+) cations (0.1-1.0 mM) strongly stimulated all its DNA repair activities. Optimization of the reaction conditions revealed that the wheat enzyme requires low divalent cation concentration (0.1 mM), mildly acidic pH (6-7), low ionic strength (20 mM KCl) and has a temperature optimum at around 20 °C. The steady-state kinetic parameters of enzymatic reactions indicate that TaApe1L removes 3'-blocking sugar-phosphate and 3'-phosphate groups with good efficiency (kcat/KM = 630 and 485 μM(-1) · min(-1), respectively) but possesses a very weak AP endonuclease activity as compared to the human homologue, APE1.
Conclusions/significance: Taken together, these data establish the DNA substrate specificity of the wheat AP endonuclease and suggest its possible role in the repair of DNA damage generated by endogenous and environmental factors.
Conflict of interest statement
Figures





References
-
- Kavli B, Otterlei M, Slupphaug G, Krokan HE (2007) Uracil in DNA—general mutagen, but normal intermediate in acquired immunity. DNA Repair (Amst) 6: 505–516. - PubMed
-
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, et al. (2006) DNA repair and mutagenesis: ASM Press.
-
- Cadet J, Douki T, Gasparutto D, Ravanat JL (2003) Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res 531: 5–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

