Efficacy of two versus three-day regimens of dihydroartemisinin-piperaquine for uncomplicated malaria in military personnel in northern Cambodia: an open-label randomized trial
- PMID: 24667662
- PMCID: PMC3965521
- DOI: 10.1371/journal.pone.0093138
Efficacy of two versus three-day regimens of dihydroartemisinin-piperaquine for uncomplicated malaria in military personnel in northern Cambodia: an open-label randomized trial
Abstract
Introduction: Emerging antimalarial drug resistance in mobile populations remains a significant public health concern. We compared two regimens of dihydroartemisinin-piperaquine in military and civilians on the Thai-Cambodian border to evaluate national treatment policy.
Methods: Efficacy and safety of two and three-day regimens of dihydroartemisinin-piperaquine were compared as a nested open-label evaluation within a malaria cohort study in 222 otherwise healthy volunteers (18% malaria-infected at baseline). The first 80 volunteers with slide-confirmed Plasmodium falciparum or vivax malaria were randomized 1:1 to receive either regimen (total dose 360 mg dihydroartemisinin and 2880 mg piperaquine) and followed weekly for up to 6 months. The primary endpoint was malaria recurrence by day 42. Volunteers with vivax infection received primaquine at study discharge with six months follow-up.
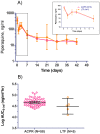
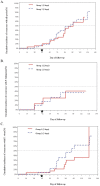
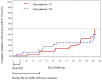
Results: Eighty patients (60 vivax, 15 falciparum, and 5 mixed) were randomized to dihydroartemisinin-piperaquine. Intention-to-treat all-species efficacy at Day 42 was 85% for the two-day regimen (95% CI 69-94) and 90% for the three-day regimen (95% CI 75-97). PCR-adjusted falciparum efficacy was 75% in both groups with nearly half (45%) still parasitemic at Day 3. Plasma piperaquine levels were comparable to prior published reports, but on the day of recrudescence were below measurable in vitro piperaquine IC50 levels in all falciparum treatment failures.
Conclusions: In the brief period since introduction of dihydroartemisinin-piperaquine, there is early evidence suggesting declining efficacy relative to previous reports. Parasite IC50 levels in excess of plasma piperaquine levels seen only in treatment failures raises concern for clinically significant piperaquine resistance in Cambodia. These findings warrant improved monitoring of clinical outcomes and follow-up, given few available alternative drugs.
Trial registration: ClinicalTrials.gov NCT01280162.
Conflict of interest statement
Figures


References
-
- World Health Organization. Global Malaria Programme (2012) World malaria report 2012. Geneva: World Health Organization. Available: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr201.... Accessed 5 February 2013.
-
- World Health Organization Global Malaria Programme (n.d.) Update on artemisinin resistance–April 2012. Available: http://www.who.int/malaria/publications/atoz/arupdate042012. pdf. Accessed 8 February 2013.
-
- Tarning J, Ashley EA, Lindegardh N, Stepniewska K, Phaiphun L, et al. (2008) Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother 52: 1052–1061 10.1128/AAC.0095507 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

