High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: results from the EORTC 10041/BIG 03-04 MINDACT trial
- PMID: 24667714
- PMCID: PMC3969556
- DOI: 10.1093/annonc/mdu026
High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: results from the EORTC 10041/BIG 03-04 MINDACT trial
Abstract
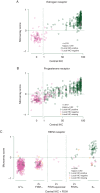
Background: To investigate the correlation of TargetPrint with local and central immunohistochemistry/fluorescence in situ hybridization assessment of estrogen (ER), progesterone (PgR), and human epidermal growth factor receptor 2 (HER2) in the first 800 patients enrolled in the MINDACT trial.
Patients and methods: Data from local (N = 800) and central (N = 626) assessments of receptor status were collected and compared with TargetPrint results.
Results: For ER, the positive agreement (the percentage of central pathology positive assessments that were also TargetPrint/local laboratory positive) for TargetPrint in comparison to centralized assessment was 98% with a negative agreement (the percentage of central pathology negative assessments that were also TargetPrint/local laboratory negative) of 96%. For PgR, the positive agreement was 83% with a negative agreement of 92%. For HER2, the positive agreement was 75% with a negative agreement of 99%. Even though the local assessment showed higher positive agreement for PgR (89%) and higher positive agreement for HER2 (85%), the range of discordant local versus central assessments were as high as 6.7% for ER, 12.9% for PgR, and 4.3% for HER2.
Conclusion: TargetPrint and local assessment of ER, PgR, and HER2 show high concordance with central assessment in the first 800 MINDACT patients. However, there are concerns about the higher discordance rates for some local sites. TargetPrint can improve the reliability of hormone receptor and HER2 testing for those centers with a lower rate of concordance with the reference laboratory, with the limitation of a positive agreement of 75% for HER2. TargetPrint consequently has important implications for treatment decisions in clinical practice and is a reliable alternative to local assessment for ER.
Clinical trials number: NCT00433589.
Keywords: FISH; IHC; TargetPrint; breast cancer; concordance; hormone receptor.
Figures

References
-
- Rutgers E, Piccart-Gebhart MJ, Bogaerts J, et al. The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;00:2742–2749. - PubMed
-
- Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi12–vi24. - PubMed
-
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. 2013. Breast Cancer. Version 2.2013 http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. (23 December 2013, date last accessed) - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

