Telmisartan induces growth inhibition, DNA double-strand breaks and apoptosis in human endometrial cancer cells
- PMID: 24667764
- PMCID: PMC3965508
- DOI: 10.1371/journal.pone.0093050
Telmisartan induces growth inhibition, DNA double-strand breaks and apoptosis in human endometrial cancer cells
Abstract
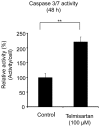
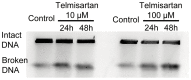
Telmisartan, an angiotensin II receptor type 1 blocker, is often used as an antihypertension drug, and it has also been characterized as a peroxisome proliferator-activated receptor-gamma (PPARγ) ligand. The purpose of this study was to elucidate the antitumor effects of telmisartan on endometrial cancer cells. We treated three endometrial cancer cell lines with various concentrations of telmisartan, and we investigated the effects of the telmisartan on the cell proliferation, apoptosis, and their related measurements in vitro. We also administered telmisartan to nude mice with experimental tumors to determine its in vivo effects and toxicity. All three endometrial cancer cell lines were sensitive to the growth-inhibitory effect of telmisartan. The induction of apoptosis was confirmed in concert with the altered expression of genes and proteins related to the apoptosis. We also observed that DNA double-strand breaks (DSBs) were induced in HHUA (human endometrial cancer) cells by telmisartan treatment. In addition, experiments in nude mice showed that telmisartan significantly inhibited human endometrial tumor growth, without toxic side effects. Our results suggest that telmisartan might be a new therapeutic option for the treatment of endometrial cancers.
Conflict of interest statement
Figures





Similar articles
-
The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKα/mTOR pathway in vitro and in vivo.Oncotarget. 2017 Jan 31;8(5):8536-8549. doi: 10.18632/oncotarget.14345. Oncotarget. 2017. PMID: 28052030 Free PMC article.
-
Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest.Int J Oncol. 2017 Dec;51(6):1674-1684. doi: 10.3892/ijo.2017.4177. Epub 2017 Oct 23. Int J Oncol. 2017. PMID: 29075786 Free PMC article.
-
Telmisartan is a potent target for prevention and treatment in human prostate cancer.Oncol Rep. 2008 Aug;20(2):295-300. Oncol Rep. 2008. PMID: 18636189
-
Telmisartan exerts anti-tumor effects by activating peroxisome proliferator-activated receptor-γ in human lung adenocarcinoma A549 cells.Molecules. 2014 Mar 5;19(3):2862-76. doi: 10.3390/molecules19032862. Molecules. 2014. PMID: 24603556 Free PMC article.
-
Increase of human prostate cancer cell (DU145) apoptosis by telmisartan through PPAR-delta pathway.Eur J Pharmacol. 2016 Mar 15;775:35-42. doi: 10.1016/j.ejphar.2016.02.017. Epub 2016 Feb 4. Eur J Pharmacol. 2016. PMID: 26852954
Cited by
-
Telmisartan inhibits hepatocellular carcinoma cell proliferation in vitro by inducing cell cycle arrest.Oncol Rep. 2017 Nov;38(5):2825-2835. doi: 10.3892/or.2017.5977. Epub 2017 Sep 20. Oncol Rep. 2017. PMID: 29048654 Free PMC article.
-
Telmisartan attenuates N-nitrosodiethylamine-induced hepatocellular carcinoma in mice by modulating the NF-κB-TAK1-ERK1/2 axis in the context of PPARγ agonistic activity.Naunyn Schmiedebergs Arch Pharmacol. 2019 Dec;392(12):1591-1604. doi: 10.1007/s00210-019-01706-2. Epub 2019 Jul 31. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 31367864
-
MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen.Cancers (Basel). 2022 May 23;14(10):2563. doi: 10.3390/cancers14102563. Cancers (Basel). 2022. PMID: 35626167 Free PMC article.
-
LY333531, a PKCβ inhibitor, attenuates glomerular endothelial cell apoptosis in the early stage of mouse diabetic nephropathy via down-regulating swiprosin-1.Acta Pharmacol Sin. 2017 Jul;38(7):1009-1023. doi: 10.1038/aps.2016.172. Epub 2017 Apr 17. Acta Pharmacol Sin. 2017. PMID: 28414198 Free PMC article.
-
Repurposed Drugs in Gastric Cancer.Molecules. 2022 Dec 30;28(1):319. doi: 10.3390/molecules28010319. Molecules. 2022. PMID: 36615513 Free PMC article. Review.
References
-
- Obel JC, Friberg G, Fleming GF (2006) Chemotherapy in endometrial cancer. Clin Adv Hematol Oncol 4: 459–468. - PubMed
-
- Ota K, Ito K, Suzuki T, Saito S, Tamura M, et al. (2006) Peroxisome proliferator-activated receptor gamma and growth inhibition by its ligands in uterine endometrial carcinoma. Clin Cancer Res 12: 4200–4208. - PubMed
-
- Xin B, Yokoyama Y, Shigeto T, Futagami M, Mizunuma H (2007) Inhibitory effect of meloxicam, a selective cyclooxygenase-2 inhibitor, and ciglitazone, a peroxisome proliferator-activated receptor gamma ligand, on the growth of human ovarian cancers. Cancer 110: 791–800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

