11β-hydroxysteroid dehydrogenase 1 specific inhibitor increased dermal collagen content and promotes fibroblast proliferation
- PMID: 24667799
- PMCID: PMC3965512
- DOI: 10.1371/journal.pone.0093051
11β-hydroxysteroid dehydrogenase 1 specific inhibitor increased dermal collagen content and promotes fibroblast proliferation
Abstract
Glucocorticoids (GCs) are one of the most effective anti-inflammatory drugs for treating acute and chronic inflammatory diseases. However, several studies have shown that GCs alter collagen metabolism in the skin and induce skin atrophy. Cortisol is the endogenous GC that is released in response to various stressors. Over the last decade, extraadrenal cortisol production in various tissues has been reported. Skin also synthesizes cortisol through a de novo pathway and through an activating enzyme. 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) is the enzyme that catalyzes the conversion of hormonally inactive cortisone to active cortisol in cells. We previously found that 11β-HSD1 negatively regulates proliferation of keratinocytes. To determine the function of 11β-HSD1 in dermal fibroblasts and collagen metabolism, the effect of a selective 11β-HSD1 inhibitor was studied in mouse tissues and dermal fibroblasts. The expression of 11β-HSD1 increased with age in mouse skin. Subcutaneous injection of a selective 11β-HSD1 inhibitor increased dermal thickness and collagen content in the mouse skin. In vitro, proliferation of dermal fibroblasts derived from 11β-HSD1 null mice (Hsd11b1(-/-) mice) was significantly increased compared with fibroblasts from wild-type mice. However, in vivo, dermal thickness of Hsd11b1(-/-) mice was not altered in 3-month-old and 1-year-old mouse skin compared with wild-type mouse skin. These in vivo findings suggest the presence of compensatory mechanisms in Hsd11b1(-/-) mice. Our findings suggest that 11β-HSD1 inhibition may reverse the decreased collagen content observed in intrinsically and extrinsically aged skin and in skin atrophy that is induced by GC treatment.
Conflict of interest statement
Figures
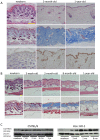
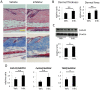

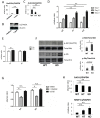
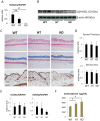
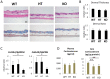
References
-
- Chebotaev D, Yemelyanov A, Budunova I (2007) The mechanisms of tumor suppressor effect of glucocorticoid receptor in skin. Mol Carcinog 46: 732–740. - PubMed
-
- Chebotaev DV, Yemelyanov AY, Lavker RM, Budunova IV (2007) Epithelial cells in the hair follicle bulge do not contribute to epidermal regeneration after glucocorticoid-induced cutaneous atrophy. J Invest Dermatol 127: 2749–2758. - PubMed
-
- Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T (2002) Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br J Dermatol 147: 859–868. - PubMed
-
- Nuutinen P, Autio P, Hurskainen T, Oikarinen A (2001) Glucocorticoid action on skin collagen: overview on clinical significance and consequences. J Eur Acad Dermatol Venereol 15: 361–362. - PubMed
-
- Averbeck M, Gebhardt C, Anderegg U, Simon JC (2010) Suppression of hyaluronan synthase 2 expression reflects the atrophogenic potential of glucocorticoids. Exp Dermatol 19: 757–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

