Skeletal muscle DNA damage precedes spinal motor neuron DNA damage in a mouse model of Spinal Muscular Atrophy (SMA)
- PMID: 24667816
- PMCID: PMC3965546
- DOI: 10.1371/journal.pone.0093329
Skeletal muscle DNA damage precedes spinal motor neuron DNA damage in a mouse model of Spinal Muscular Atrophy (SMA)
Abstract
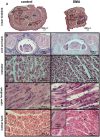
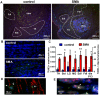
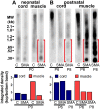
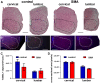
Spinal Muscular Atrophy (SMA) is a hereditary childhood disease that causes paralysis by progressive degeneration of skeletal muscles and spinal motor neurons. SMA is associated with reduced levels of full-length Survival of Motor Neuron (SMN) protein, due to mutations in the Survival of Motor Neuron 1 gene. The mechanisms by which lack of SMN causes SMA pathology are not known, making it very difficult to develop effective therapies. We investigated whether DNA damage is a perinatal pathological event in SMA, and whether DNA damage and cell death first occur in skeletal muscle or spinal cord of SMA mice. We used a mouse model of severe SMA to ascertain the extent of cell death and DNA damage throughout the body of prenatal and newborn mice. SMA mice at birth (postnatal day 0) exhibited internucleosomal fragmentation in genomic DNA from hindlimb skeletal muscle, but not in genomic DNA from spinal cord. SMA mice at postnatal day 5, compared with littermate controls, exhibited increased apoptotic cell death profiles in skeletal muscle, by hematoxylin and eosin, terminal deoxynucleotidyl transferase dUTP nick end labeling, and electron microscopy. SMA mice had no increased cell death, no loss of choline acetyl transferase (ChAT)-positive motor neurons, and no overt pathology in the ventral horn of the spinal cord. At embryonic days 13 and 15.5, SMA mice did not exhibit statistically significant increases in cell death profiles in spinal cord or skeletal muscle. Motor neuron numbers in the ventral horn, as identified by ChAT immunoreactivity, were comparable in SMA mice and control littermates at embryonic day 15.5 and postnatal day 5. These observations demonstrate that in SMA, disease in skeletal muscle emerges before pathology in spinal cord, including loss of motor neurons. Overall, this work identifies DNA damage and cell death in skeletal muscle as therapeutic targets for SMA.
Conflict of interest statement
Figures




Similar articles
-
Spinal motor neuron loss occurs through a p53-and-p21-independent mechanism in the Smn2B/- mouse model of spinal muscular atrophy.Exp Neurol. 2021 Mar;337:113587. doi: 10.1016/j.expneurol.2020.113587. Epub 2020 Dec 28. Exp Neurol. 2021. PMID: 33382987 Free PMC article.
-
Chronic treatment with lithium does not improve neuromuscular phenotype in a mouse model of severe spinal muscular atrophy.Neuroscience. 2013 Oct 10;250:417-33. doi: 10.1016/j.neuroscience.2013.07.026. Epub 2013 Jul 19. Neuroscience. 2013. PMID: 23876328
-
Fasudil improves survival and promotes skeletal muscle development in a mouse model of spinal muscular atrophy.BMC Med. 2012 Mar 7;10:24. doi: 10.1186/1741-7015-10-24. BMC Med. 2012. PMID: 22397316 Free PMC article.
-
Spinal Muscular Atrophy: More than a Disease of Motor Neurons?Curr Mol Med. 2016;16(9):779-792. doi: 10.2174/1566524016666161128113338. Curr Mol Med. 2016. PMID: 27894243 Review.
-
Spinal muscular atrophy and the antiapoptotic role of survival of motor neuron (SMN) protein.Mol Neurobiol. 2013 Apr;47(2):821-32. doi: 10.1007/s12035-013-8399-5. Epub 2013 Jan 13. Mol Neurobiol. 2013. PMID: 23315303 Review.
Cited by
-
Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients.Int J Mol Sci. 2021 May 26;22(11):5673. doi: 10.3390/ijms22115673. Int J Mol Sci. 2021. PMID: 34073630 Free PMC article.
-
Chronic Treatment with the AMPK Agonist AICAR Prevents Skeletal Muscle Pathology but Fails to Improve Clinical Outcome in a Mouse Model of Severe Spinal Muscular Atrophy.Neurotherapeutics. 2016 Jan;13(1):198-216. doi: 10.1007/s13311-015-0399-x. Neurotherapeutics. 2016. PMID: 26582176 Free PMC article.
-
The Cajal Body Protein WRAP53β Prepares the Scene for Repair of DNA Double-Strand Breaks by Regulating Local Ubiquitination.Front Mol Biosci. 2019 Jul 4;6:51. doi: 10.3389/fmolb.2019.00051. eCollection 2019. Front Mol Biosci. 2019. PMID: 31334247 Free PMC article. Review.
-
R-loop Mediated DNA Damage and Impaired DNA Repair in Spinal Muscular Atrophy.Front Cell Neurosci. 2022 Jun 16;16:826608. doi: 10.3389/fncel.2022.826608. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35783101 Free PMC article. Review.
-
Gender-Specific Amelioration of SMA Phenotype upon Disruption of a Deep Intronic Structure by an Oligonucleotide.Mol Ther. 2017 Jun 7;25(6):1328-1341. doi: 10.1016/j.ymthe.2017.03.036. Epub 2017 Apr 13. Mol Ther. 2017. PMID: 28412171 Free PMC article.
References
-
- MacLeod MJ, Taylor JE, Lunt PW, Mathew CG, Robb SA (1999) Prenatal onset spinal muscular atrophy. Eur J Paediatr Neurol 3: 65–72. - PubMed
-
- Crawford TO, Pardo CA (1996) The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis 3: 97–110. - PubMed
-
- Thomas NH, Dubowitz V (1994) The natural history of type I (severe) spinal muscular atrophy. Neuromuscul Disord 4: 497–502. - PubMed
-
- Dubowitz V (1964) Infantile muscular atrophy. A prospective study with particular reference to a slowly progressive variety. Brain 87: 707–718. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

