Differential effects of Tat proteins derived from HIV-1 subtypes B and recombinant CRF02_AG on human brain microvascular endothelial cells: implications for blood-brain barrier dysfunction
- PMID: 24667918
- PMCID: PMC4050250
- DOI: 10.1038/jcbfm.2014.54
Differential effects of Tat proteins derived from HIV-1 subtypes B and recombinant CRF02_AG on human brain microvascular endothelial cells: implications for blood-brain barrier dysfunction
Abstract
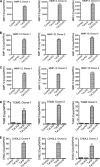
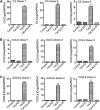
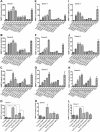
HIV-1 genetic differences influence viral replication and progression to AIDS. HIV-1 circulating recombinant form (CRF)02_AG is the predominant viral subtype infecting humans in West and Central Africa, but its effects on HIV neuropathogenesis are not known. In the present study, we investigated the effects of Tat proteins from HIV-1 subtype B (Tat.B) and HIV-1 CRF02_AG (Tat.AG) on primary human brain microvascular endothelial cells (HBMEC), the major component of the blood-brain barrier (BBB). Using Affymetrix GeneChip Human Gene 1.0.ST arrays, we showed that Tat.AG had minimal effects while Tat.B induced transcriptional upregulation of 90 genes in HBMEC, including proinflammatory chemokines, complement components C3, C7, and complement factor B, matrix metalloproteinases (MMP)-3, MMP-10, and MMP-12. These results were confirmed by real-time PCR. Compared with Tat.AG, Tat.B significantly increased MMP-3, MMP-10, and MMP-12 activities in HBMEC, and the MMPs tissue inhibitor of metalloproteinase-2 blocked Tat-induced increase in MMPs activity. Western blot analyses also showed that Tat increased the expression of C3 and its cleaved fragment C3b in HBMEC. These data suggest that genetic differences between HIV-1 subtypes B and CRF02_AG influence the effects of Tat proteins from these two clades on HBMEC, including molecular and cellular functions, and canonical pathways, which would affect BBB dysfunction and viral neuropathogenesis.
Figures



Similar articles
-
Differential Mechanisms of Inflammation and Endothelial Dysfunction by HIV-1 Subtype-B and Recombinant CRF02_AG Tat Proteins on Human Brain Microvascular Endothelial Cells: Implications for Viral Neuropathogenesis.Mol Neurobiol. 2018 Feb;55(2):1352-1363. doi: 10.1007/s12035-017-0382-0. Epub 2017 Jan 27. Mol Neurobiol. 2018. PMID: 28127697 Free PMC article.
-
HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9.Brain Res. 2012 Feb 3;1436:13-9. doi: 10.1016/j.brainres.2011.11.052. Epub 2011 Dec 7. Brain Res. 2012. PMID: 22197032
-
Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder.J Neurovirol. 2010 Jul;16(4):294-305. doi: 10.3109/13550284.2010.499891. J Neurovirol. 2010. PMID: 20624003
-
Disruption of blood-brain barrier: effects of HIV Tat on brain microvascular endothelial cells and tight junction proteins.J Neurovirol. 2023 Dec;29(6):658-668. doi: 10.1007/s13365-023-01179-3. Epub 2023 Oct 29. J Neurovirol. 2023. PMID: 37899420 Review.
-
Signatures of HIV-1 subtype B and C Tat proteins and their effects in the neuropathogenesis of HIV-associated neurocognitive impairments.Neurobiol Dis. 2020 Mar;136:104701. doi: 10.1016/j.nbd.2019.104701. Epub 2019 Dec 11. Neurobiol Dis. 2020. PMID: 31837421 Review.
Cited by
-
Effects of HIV on executive function and verbal fluency in Cameroon.Sci Rep. 2018 Dec 12;8(1):17794. doi: 10.1038/s41598-018-36193-7. Sci Rep. 2018. PMID: 30542105 Free PMC article.
-
Functional impact of HIV-1 Tat on cells of the CNS and its role in HAND.Cell Mol Life Sci. 2020 Dec;77(24):5079-5099. doi: 10.1007/s00018-020-03561-4. Epub 2020 Jun 23. Cell Mol Life Sci. 2020. PMID: 32577796 Free PMC article. Review.
-
SERPINA3: Stimulator or Inhibitor of Pathological Changes.Biomedicines. 2023 Jan 7;11(1):156. doi: 10.3390/biomedicines11010156. Biomedicines. 2023. PMID: 36672665 Free PMC article. Review.
-
Promoter polymorphism MMP-1 (-1607 2G/1G) and MMP-3 (-1612 5A/6A) in development of HAND and modulation of pathogenesis of HAND.J Biosci. 2017 Sep;42(3):481-490. doi: 10.1007/s12038-017-9694-5. J Biosci. 2017. PMID: 29358561
-
Immunohistochemical Expression of the SERPINA3 Protein in Uterine Fibroids.Curr Pharm Biotechnol. 2024;25(13):1758-1765. doi: 10.2174/0113892010264673231111082438. Curr Pharm Biotechnol. 2024. PMID: 38204235
References
-
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. - PubMed
-
- Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. - PubMed
-
- Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4:259–266. - PubMed
-
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

