Role of receptor binding specificity in influenza A virus transmission and pathogenesis
- PMID: 24668228
- PMCID: PMC4194109
- DOI: 10.1002/embj.201387442
Role of receptor binding specificity in influenza A virus transmission and pathogenesis
Erratum in
- EMBO J. 2014 Jul 17;33(14):1614
Abstract
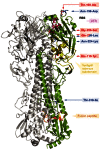
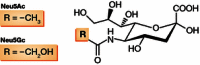
The recent emergence of a novel avian A/H7N9 influenza virus in poultry and humans in China, as well as laboratory studies on adaptation and transmission of avian A/H5N1 influenza viruses, has shed new light on influenza virus adaptation to mammals. One of the biological traits required for animal influenza viruses to cross the species barrier that received considerable attention in animal model studies, in vitro assays, and structural analyses is receptor binding specificity. Sialylated glycans present on the apical surface of host cells can function as receptors for the influenza virus hemagglutinin (HA) protein. Avian and human influenza viruses typically have a different sialic acid (SA)-binding preference and only few amino acid changes in the HA protein can cause a switch from avian to human receptor specificity. Recent experiments using glycan arrays, virus histochemistry, animal models, and structural analyses of HA have added a wealth of knowledge on receptor binding specificity. Here, we review recent data on the interaction between influenza virus HA and SA receptors of the host, and the impact on virus host range, pathogenesis, and transmission. Remaining challenges and future research priorities are also discussed.
Figures

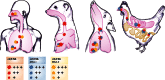

References
-
- Ablan S, Rawat SS, Blumenthal R, Puri A (2001) Entry of influenza virus into a glycosphingolipid‐deficient mouse skin fibroblast cell line. Arch Virol 146: 2227–2238 - PubMed
-
- Almond JW (1977) A single gene determines the host range of influenza virus. Nature 270: 617–618 - PubMed
-
- Baum LG, Paulson JC (1991) The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology 180: 10–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

