Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912
- PMID: 24668328
- PMCID: PMC3983816
- DOI: 10.1634/theoncologist.2013-0306
Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912
Abstract
Background: In advanced gastric cancer (AGC), no globally accepted prognostic scoring system has been developed. Therefore, we explored baseline prognostic factors in Japanese AGC patients using the data from a randomized controlled trial, Japan Clinical Oncology Group (JCOG) 9912, which investigated the efficacy of systemic chemotherapy as a first-line treatment.
Patients and methods: Prognostic factors and prognostic indices for overall survival were screened and evaluated in patients enrolled in JCOG9912 using the Cox proportional hazard model. The Royal Marsden Hospital prognostic model was also applied to the JCOG9912 trial.
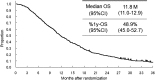
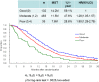
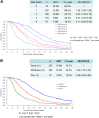
Results: A total of 650 (92.3%) of the 704 patients randomized in the JCOG9912 trial, for whom complete data were available for multivariate analyses, was included in the present study (5-fluorouracil arm, n = 215; irinotecan plus cisplatin arm, n = 216; S-1 arm, n = 219). The median survival time (MST) for all patients was 11.8 months. To construct a prognostic index, we selected four risk factors by multivariate analysis: performance status ≥ 1, number of metastatic sites ≥ 2, no prior gastrectomy, and elevated alkaline phosphatase. MSTs were 17.0 months for patients categorized into the low-risk group, who had zero or one risk factor (n = 225); 10.4 months for patients in the moderate-risk group, who had two or three risk factors (n = 368); and 5.0 months for patients in the high-risk group, who had all four risk factors (n = 57).
Conclusion: In the present study, we propose a new prognostic index for patients with AGC. This can be used for more appropriate patient stratification in future clinical trials.
Trial registration: ClinicalTrials.gov NCT00142350.
Keywords: Advanced gastric cancer; Chemotherapy; Prognostic factor; Prognostic index.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. - PubMed
-
- Glimelius B, Hoffman K, Haglund U, et al. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994;5:189–190. - PubMed
-
- Cunningham D, Starling N, Rao S, et al. Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
-
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

