A microRNA processing defect in smokers' macrophages is linked to SUMOylation of the endonuclease DICER
- PMID: 24668803
- PMCID: PMC4007470
- DOI: 10.1074/jbc.M114.565473
A microRNA processing defect in smokers' macrophages is linked to SUMOylation of the endonuclease DICER
Abstract
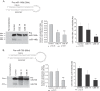
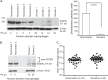
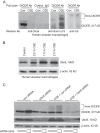
Despite the fact that alveolar macrophages play an important role in smoking-related disease, little is known about what regulates their pathophysiologic phenotype. Evaluating smoker macrophages, we found significant down-regulation of multiple microRNAs (miRNAs). This work investigates the hypothesis that cigarette smoke alters mature miRNA expression in lung macrophages by inhibiting processing of primary miRNA transcripts. Studies on smoker alveolar macrophages showed a defect in miRNA maturation. Studies on the miRNA biogenesis machinery led us to focus on the cytosolic RNA endonuclease, DICER. DICER cleaves the stem-loop structure from pre-miRNAs, allowing them to dissociate into their mature 20-22-nucleotide single-stranded form. DICER activity assays confirmed impaired DICER activity following cigarette smoke exposure. Further protein studies demonstrated a decreased expression of the native 217-kDa form of DICER and an accumulation of high molecular weight forms with cigarette smoke exposure. This molecular mass shift was shown to contain SUMO moieties and could be blocked by silencing RNA directed at the primary SUMOylating ligase, Ubc9. In determining the cigarette smoke components responsible for changes in DICER, we found that N-acetylcysteine, an antioxidant and anti-aldehyde, protected DICER protein and activity from cigarette smoke extract. This massive down-regulation of miRNAs (driven in part by alterations in DICER) may be an important regulator of the disease-promoting macrophage phenotype found in the lungs of smokers.
Keywords: Chronic Obstructive Pulmonary Disease (COPD); Cigarette Smoke; DICER; Epigenetics; Macrophage; MicroRNA (miRNA); SUMOylation; Small Ubiquitin-like Modifier (SUMO).
Figures




References
-
- Martin T. R., Raghu G., Maunder R. J., Springmeyer S. C. (1985) The effects of chronic bronchitis and chronic air-flow obstruction on lung cell populations recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 132, 254–260 - PubMed
-
- Shapiro S. D., Ingenito E. P. (2005) The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am. J. Respir. Cell Mol. Biol. 32, 367–372 - PubMed
-
- Tetley T. D. (2002) Macrophages and the pathogenesis of COPD. Chest 121, 156S–159S - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

