Locus of enterocyte effacement-encoded regulator (Ler) of pathogenic Escherichia coli competes off histone-like nucleoid-structuring protein (H-NS) through noncooperative DNA binding
- PMID: 24668810
- PMCID: PMC4022848
- DOI: 10.1074/jbc.M113.545954
Locus of enterocyte effacement-encoded regulator (Ler) of pathogenic Escherichia coli competes off histone-like nucleoid-structuring protein (H-NS) through noncooperative DNA binding
Abstract
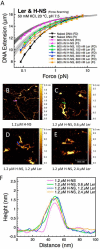
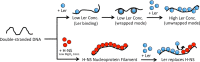
The locus of enterocyte effacement-encoded regulator (Ler) of enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) functions to activate transcription of virulence genes silenced by the histone-like nucleoid-structuring protein (H-NS). Despite its important role in the bacterial gene regulation, the binding mode of Ler to DNA and its mechanism in alleviating genes repressed by H-NS are largely unknown. In this study, we use magnetic tweezers to demonstrate that Ler binds extended DNA through a largely noncooperative process, which results in DNA stiffening and DNA folding depending on protein concentration. We also show that Ler can replace prebound H-NS on DNA over a range of potassium and magnesium concentrations. Our findings reveal the DNA binding properties of Ler and shed light to further understand the anti-silencing activity of Ler.
Keywords: Atomic Force Microscopy; DNA-binding Protein; Gene Regulation; H-NS; Ler; Magnetic Tweezers; Protein-DNA Interaction; Single Molecule Biophysics.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Bacterial-Chromatin Structural Proteins Regulate the Bimodal Expression of the Locus of Enterocyte Effacement (LEE) Pathogenicity Island in Enteropathogenic Escherichia coli.mBio. 2017 Aug 8;8(4):e00773-17. doi: 10.1128/mBio.00773-17. mBio. 2017. PMID: 28790204 Free PMC article.
-
Mutational analysis of the locus of enterocyte effacement-encoded regulator (Ler) of enteropathogenic Escherichia coli.J Bacteriol. 2008 Dec;190(23):7808-18. doi: 10.1128/JB.00663-08. Epub 2008 Oct 3. J Bacteriol. 2008. PMID: 18835988 Free PMC article.
-
DNA looping-dependent autorepression of LEE1 P1 promoters by Ler in enteropathogenic Escherichia coli (EPEC).Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):E2586-95. doi: 10.1073/pnas.1322033111. Epub 2014 Jun 11. Proc Natl Acad Sci U S A. 2014. PMID: 24920590 Free PMC article.
-
Tight Junction Disruption Induced by Type 3 Secretion System Effectors Injected by Enteropathogenic and Enterohemorrhagic Escherichia coli.Front Cell Infect Microbiol. 2016 Aug 24;6:87. doi: 10.3389/fcimb.2016.00087. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27606286 Free PMC article. Review.
-
The Tip of the Iceberg: On the Roles of Regulatory Small RNAs in the Virulence of Enterohemorrhagic and Enteropathogenic Escherichia coli.Front Cell Infect Microbiol. 2016 Sep 21;6:105. doi: 10.3389/fcimb.2016.00105. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27709103 Free PMC article. Review.
Cited by
-
Single-molecule study on histone-like nucleoid-structuring protein (H-NS) paralogue in Pseudomonas aeruginosa: MvaU bears DNA organization mode similarities to MvaT.PLoS One. 2014 Nov 5;9(11):e112246. doi: 10.1371/journal.pone.0112246. eCollection 2014. PLoS One. 2014. PMID: 25372370 Free PMC article.
-
Enteropathogens: Tuning Their Gene Expression for Hassle-Free Survival.Front Microbiol. 2019 Jan 9;9:3303. doi: 10.3389/fmicb.2018.03303. eCollection 2018. Front Microbiol. 2019. PMID: 30687282 Free PMC article. Review.
-
Dimerization site 2 of the bacterial DNA-binding protein H-NS is required for gene silencing and stiffened nucleoprotein filament formation.J Biol Chem. 2018 Jun 15;293(24):9496-9505. doi: 10.1074/jbc.RA117.001425. Epub 2018 Apr 25. J Biol Chem. 2018. PMID: 29695505 Free PMC article.
-
H-NS Regulates Gene Expression and Compacts the Nucleoid: Insights from Single-Molecule Experiments.Biophys J. 2015 Oct 6;109(7):1321-9. doi: 10.1016/j.bpj.2015.08.016. Biophys J. 2015. PMID: 26445432 Free PMC article. Review.
-
Engineering the Controlled Assembly of Filamentous Injectisomes in E. coli K-12 for Protein Translocation into Mammalian Cells.ACS Synth Biol. 2015 Sep 18;4(9):1030-41. doi: 10.1021/acssynbio.5b00080. Epub 2015 Jun 12. ACS Synth Biol. 2015. PMID: 26017572 Free PMC article.
References
-
- Dorman C. (2004) H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2, 391–400 - PubMed
-
- Ali S. S., Xia B., Liu J., Navarre W. W. (2012) Silencing of foreign DNA in bacteria. Curr. Opin. Microbiol. 15, 175–181 - PubMed
-
- Torres A. G., López-Sánchez G. N., Milflores-Flores L., Patel S. D., Rojas-López M., Martínez de la Peña C. F., Arenas-Hernández M. M., Martínez-Laguna Y. (2007) Ler and H-NS, regulators controlling expression of the long polar fimbriae of Escherichia coli O157:H7. J. Bacteriol. 189, 5916–5928 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

