Z-disc-associated, alternatively spliced, PDZ motif-containing protein (ZASP) mutations in the actin-binding domain cause disruption of skeletal muscle actin filaments in myofibrillar myopathy
- PMID: 24668811
- PMCID: PMC4036366
- DOI: 10.1074/jbc.M114.550418
Z-disc-associated, alternatively spliced, PDZ motif-containing protein (ZASP) mutations in the actin-binding domain cause disruption of skeletal muscle actin filaments in myofibrillar myopathy
Abstract
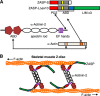
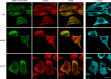
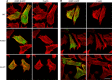
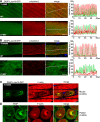
The core of skeletal muscle Z-discs consists of actin filaments from adjacent sarcomeres that are cross-linked by α-actinin homodimers. Z-disc-associated, alternatively spliced, PDZ motif-containing protein (ZASP)/Cypher interacts with α-actinin, myotilin, and other Z-disc proteins via the PDZ domain. However, these interactions are not sufficient to maintain the Z-disc structure. We show that ZASP directly interacts with skeletal actin filaments. The actin-binding domain is between the modular PDZ and LIM domains. This ZASP region is alternatively spliced so that each isoform has unique actin-binding domains. All ZASP isoforms contain the exon 6-encoded ZASP-like motif that is mutated in zaspopathy, a myofibrillar myopathy (MFM), whereas the exon 8-11 junction-encoded peptide is exclusive to the postnatal long ZASP isoform (ZASP-LΔex10). MFM is characterized by disruption of skeletal muscle Z-discs and accumulation of myofibrillar degradation products. Wild-type and mutant ZASP interact with α-actin, α-actinin, and myotilin. Expression of mutant, but not wild-type, ZASP leads to Z-disc disruption and F-actin accumulation in mouse skeletal muscle, as in MFM. Mutations in the actin-binding domain of ZASP-LΔex10, but not other isoforms, cause disruption of the actin cytoskeleton in muscle cells. These isoform-specific mutation effects highlight the essential role of the ZASP-LΔex10 isoform in F-actin organization. Our results show that MFM-associated ZASP mutations in the actin-binding domain have deleterious effects on the core structure of the Z-discs in skeletal muscle.
Keywords: Actin; Actinin; Muscular Dystrophy; Myofibrillar Myopathy; Myotilin; Protein Complex; Protein-Protein Interaction; Skeletal Muscle; Z-disc; ZASP.
Figures




Similar articles
-
Zasp52, a Core Z-disc Protein in Drosophila Indirect Flight Muscles, Interacts with α-Actinin via an Extended PDZ Domain.PLoS Genet. 2016 Oct 26;12(10):e1006400. doi: 10.1371/journal.pgen.1006400. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27783625 Free PMC article.
-
Expression and Purification of ZASP Subdomains and Clinically Important Isoforms: High-Affinity Binding to G-Actin.Biochemistry. 2017 Apr 11;56(14):2061-2070. doi: 10.1021/acs.biochem.7b00067. Epub 2017 Apr 3. Biochemistry. 2017. PMID: 28349680 Free PMC article.
-
Zasp/Cypher internal ZM-motif containing fragments are sufficient to co-localize with alpha-actinin--analysis of patient mutations.Exp Cell Res. 2006 May 1;312(8):1299-311. doi: 10.1016/j.yexcr.2005.12.036. Epub 2006 Feb 14. Exp Cell Res. 2006. PMID: 16476425
-
The Role of Z-disc Proteins in Myopathy and Cardiomyopathy.Int J Mol Sci. 2021 Mar 17;22(6):3058. doi: 10.3390/ijms22063058. Int J Mol Sci. 2021. PMID: 33802723 Free PMC article. Review.
-
Myofibrillar myopathies.Handb Clin Neurol. 2013;113:1337-42. doi: 10.1016/B978-0-444-59565-2.00005-8. Handb Clin Neurol. 2013. PMID: 23622358 Review.
Cited by
-
Association between ZASP/LDB3 Pro26Ser and Inclusion Body Myopathy.Int J Mol Sci. 2024 Jun 14;25(12):6547. doi: 10.3390/ijms25126547. Int J Mol Sci. 2024. PMID: 38928252 Free PMC article.
-
Molecular characterization of a whirlin-like protein with biomineralization-related functions from the shell of Mytilus coruscus.PLoS One. 2020 Apr 8;15(4):e0231414. doi: 10.1371/journal.pone.0231414. eCollection 2020. PLoS One. 2020. PMID: 32267882 Free PMC article.
-
Inherited cardiomyopathies.Circ J. 2014;78(10):2347-56. doi: 10.1253/circj.cj-14-0893. Epub 2014 Sep 2. Circ J. 2014. PMID: 25186923 Free PMC article. Review.
-
Evaluation of the Genetic Basis of Familial Aggregation of Pacemaker Implantation by a Large Next Generation Sequencing Panel.PLoS One. 2015 Dec 4;10(12):e0143588. doi: 10.1371/journal.pone.0143588. eCollection 2015. PLoS One. 2015. PMID: 26636822 Free PMC article.
-
Lim Domain Binding 3 (Ldb3) Identified as a Potential Marker of Cardiac Extracellular Vesicles.Int J Mol Sci. 2022 Jul 1;23(13):7374. doi: 10.3390/ijms23137374. Int J Mol Sci. 2022. PMID: 35806378 Free PMC article.
References
-
- Luther P. K. (2000) Three-dimensional structure of a vertebrate muscle Z-band: implications for titin and α-actinin binding. J. Struct. Biol. 129, 1–16 - PubMed
-
- Sanger J. M., Sanger J. W. (2008) The dynamic Z bands of striated muscle cells. Sci. Signal. 1, pe37. - PubMed
-
- Frank D., Kuhn C., Katus H. A., Frey N. (2006) The sarcomeric Z-disc: a nodal point in signalling and disease. J. Mol. Med. 84, 446–468 - PubMed
-
- Nakano S., Engel A. G., Waclawik A. J., Emslie-Smith A. M., Busis N. A. (1996) Myofibrillar myopathy with abnormal foci of desmin positivity: I: light and electron microscopy analysis of 10 cases. J. Neuropathol. Exp. Neurol. 55, 549–562 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

