Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis
- PMID: 24668812
- PMCID: PMC4036343
- DOI: 10.1074/jbc.M113.543710
Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis
Abstract
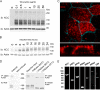
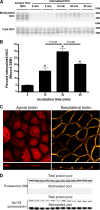
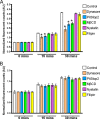
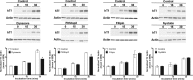
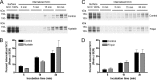
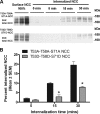
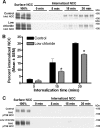
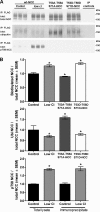
The thiazide-sensitive sodium chloride cotransporter, NCC, is the major NaCl transport protein in the distal convoluted tubule (DCT). The transport activity of NCC can be regulated by phosphorylation, but knowledge of modulation of NCC trafficking by phosphorylation is limited. In this study, we generated novel tetracycline-inducible Madin-Darby canine kidney type I (MDCKI) cell lines expressing NCC to examine the role of NCC phosphorylation and ubiquitylation on NCC endocytosis. In MDCKI-NCC cells, NCC was highly glycosylated at molecular weights consistent with NCC monomers and dimers. NCC constitutively cycles to the apical plasma membrane of MDCKI-NCC cells, with 20-30% of the membrane pool of NCC internalized within 30 min. The use of dynasore, PitStop2, methyl-β-cyclodextrin, nystatin, and filipin (specific inhibitors of either clathrin-dependent or -independent endocytosis) demonstrated that NCC is internalized via a clathrin-mediated pathway. Reduction of endocytosis resulted in greater levels of NCC in the plasma membrane. Immunogold electron microscopy confirmed the association of NCC with the clathrin-mediated internalization pathway in rat DCT cells. Compared with controls, inducing phosphorylation of NCC via low chloride treatment or mimicking phosphorylation by replacing Thr-53, Thr-58, and Ser-71 residues with Asp resulted in increased membrane abundance and reduced rates of NCC internalization. NCC ubiquitylation was lowest in the conditions with greatest NCC phosphorylation, thus providing a mechanism for the reduced endocytosis. In conclusion, our data support a model where NCC is constitutively cycled to the plasma membrane, and upon stimulation, it can be phosphorylated to both increase NCC activity and decrease NCC endocytosis, together increasing NaCl transport in the DCT.
Keywords: Endocytosis; Epithelium; Phosphorylation; Plasma Membrane; Trafficking; Ubiquitylation.
Figures
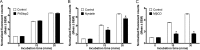



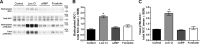
Similar articles
-
Functional assessment of sodium chloride cotransporter NCC mutants in polarized mammalian epithelial cells.Am J Physiol Renal Physiol. 2017 Aug 1;313(2):F495-F504. doi: 10.1152/ajprenal.00088.2017. Epub 2017 May 17. Am J Physiol Renal Physiol. 2017. PMID: 28515174
-
High dietary potassium causes ubiquitin-dependent degradation of the kidney sodium-chloride cotransporter.J Biol Chem. 2021 Aug;297(2):100915. doi: 10.1016/j.jbc.2021.100915. Epub 2021 Jun 24. J Biol Chem. 2021. PMID: 34174287 Free PMC article.
-
Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms.J Physiol. 2016 Nov 1;594(21):6319-6331. doi: 10.1113/JP272504. Epub 2016 Sep 11. J Physiol. 2016. PMID: 27457700 Free PMC article.
-
Regulation of the renal Na+-Cl- cotransporter by phosphorylation and ubiquitylation.Am J Physiol Renal Physiol. 2012 Dec 15;303(12):F1573-83. doi: 10.1152/ajprenal.00508.2012. Epub 2012 Oct 3. Am J Physiol Renal Physiol. 2012. PMID: 23034942 Free PMC article. Review.
-
Structure-function relationships in the renal NaCl cotransporter (NCC).Curr Top Membr. 2019;83:177-204. doi: 10.1016/bs.ctm.2019.01.003. Epub 2019 Feb 18. Curr Top Membr. 2019. PMID: 31196605 Review.
Cited by
-
WNK-Cab39-NKCC1 signaling increases the susceptibility to ischemic brain damage in hypertensive rats.J Cereb Blood Flow Metab. 2017 Aug;37(8):2780-2794. doi: 10.1177/0271678X16675368. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27798271 Free PMC article.
-
NHE3 in the thick ascending limb is required for sustained but not acute furosemide-induced urinary acidification.Am J Physiol Renal Physiol. 2022 Aug 1;323(2):F141-F155. doi: 10.1152/ajprenal.00013.2022. Epub 2022 May 30. Am J Physiol Renal Physiol. 2022. PMID: 35635321 Free PMC article.
-
Basolateral Kir4.1 activity in the distal convoluted tubule regulates K secretion by determining NaCl cotransporter activity.Curr Opin Nephrol Hypertens. 2016 Sep;25(5):429-35. doi: 10.1097/MNH.0000000000000248. Curr Opin Nephrol Hypertens. 2016. PMID: 27306796 Free PMC article. Review.
-
MAGED2 Enhances Expression and Function of NCC at the Cell Surface via cAMP Signaling Under Hypoxia.Cells. 2025 Jan 23;14(3):175. doi: 10.3390/cells14030175. Cells. 2025. PMID: 39936967 Free PMC article.
-
Cullin 3/with No Lysine [K] Kinase/Ste20/SPS-Related Proline Alanine Rich Kinase Signaling: Impact on NaCl Cotransporter Activity in BP Regulation.Kidney360. 2024 Sep 1;5(9):1386-1393. doi: 10.34067/KID.0000000000000527. Epub 2024 Aug 9. Kidney360. 2024. PMID: 39120943 Free PMC article. Review.
References
-
- Gamba G. (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 85, 423–493 - PubMed
-
- Plotkin M. D., Kaplan M. R., Verlander J. W., Lee W. S., Brown D., Poch E., Gullans S. R., Hebert S. C. (1996) Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1 in the rat kidney. Kidney Int. 50, 174–183 - PubMed
-
- Gamba G., Miyanoshita A., Lombardi M., Lytton J., Lee W. S., Hediger M. A., Hebert S. C. (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 269, 17713–17722 - PubMed
-
- de Jong J. C., Willems P. H., Mooren F. J., van den Heuvel L. P., Knoers N. V., Bindels R. J. (2003) The structural unit of the thiazide-sensitive NaCl cotransporter is a homodimer. J. Biol. Chem. 278, 24302–24307 - PubMed
-
- Hoover R. S., Poch E., Monroy A., Vázquez N., Nishio T., Gamba G., Hebert S. C. (2003) N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na(+):Cl(−) cotransporter. J. Am. Soc. Nephrol. 14, 271–282 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

