Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine
- PMID: 24668817
- PMCID: PMC4036319
- DOI: 10.1074/jbc.M114.558924
Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine
Abstract
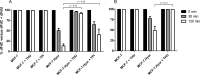
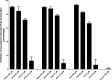
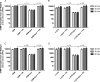
The intracellular metabolism and cytostatic activity of the anticancer drug gemcitabine (2',2'-difluoro-2'-deoxycytidine; dFdC) was severely compromised in Mycoplasma hyorhinis-infected tumor cell cultures. Pronounced deamination of dFdC to its less cytostatic metabolite 2',2'-difluoro-2'-deoxyuridine was observed, both in cell extracts and spent culture medium (i.e. tumor cell-free but mycoplasma-containing) of mycoplasma-infected tumor cells. This indicates that the decreased antiproliferative activity of dFdC in such cells is attributed to a mycoplasma cytidine deaminase causing rapid drug catabolism. Indeed, the cytostatic activity of gemcitabine could be restored by the co-administration of tetrahydrouridine (a potent cytidine deaminase inhibitor). Additionally, mycoplasma-derived pyrimidine nucleoside phosphorylase (PyNP) activity indirectly potentiated deamination of dFdC: the natural pyrimidine nucleosides uridine, 2'-deoxyuridine and thymidine inhibited mycoplasma-associated dFdC deamination but were efficiently catabolized (removed) by mycoplasma PyNP. The markedly lower anabolism and related cytostatic activity of dFdC in mycoplasma-infected tumor cells was therefore also (partially) restored by a specific TP/PyNP inhibitor (TPI), or by exogenous thymidine. Consequently, no effect on the cytostatic activity of dFdC was observed in tumor cell cultures infected with a PyNP-deficient Mycoplasma pneumoniae strain. Because it has been reported that some commensal mycoplasma species (including M. hyorhinis) preferentially colonize tumor tissue in cancer patients, our findings suggest that the presence of mycoplasmas in the tumor microenvironment could be a limiting factor for the anticancer efficiency of dFdC-based chemotherapy. Accordingly, a significantly decreased antitumor effect of dFdC was observed in mice bearing M. hyorhinis-infected murine mammary FM3A tumors compared with uninfected tumors.
Keywords: Anticancer Drug; Cancer Therapy; Cytidine Deaminase; Gemcitabine; Mycoplasma; Mycoplasma hyorhinis; Nucleoside Nucleotide Analogs; Nucleoside Nucleotide Metabolism; Phosphorylase; Pyrimidine Nucleoside Phosphorylase.
Figures

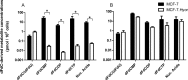



References
-
- Galmarini C. M., Mackey J. R., Dumontet C. (2002) Nucleoside analogues and nucleobases in cancer treatment. Lancet. Oncol. 3, 415–424 - PubMed
-
- Huang P., Chubb S., Hertel L. W., Grindey G. B., Plunkett W. (1991) Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 51, 6110–6117 - PubMed
-
- Ross D. D., Cuddy D. P. (1994) Molecular effects of 2′,2′-difluorodeoxycytidine (Gemcitabine) on DNA replication in intact HL-60 cells. Biochem. Pharmacol. 48, 1619–1630 - PubMed
-
- Heinemann V., Hertel L. W., Grindey G. B., Plunkett W. (1988) Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-β-d-arabinofuranosylcytosine. Cancer Res. 48, 4024–4031 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

