Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients
- PMID: 24668949
- PMCID: PMC4177031
- DOI: 10.1002/pbc.25043
Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients
Abstract
Background: Dexrazoxane may reduce anthracycline-associated cardiotoxicity in pediatric cancer patients. However, concerns of secondary acute myeloid leukemia (AML) have led to restrictions on pediatric dexrazoxane use in Europe. Published data about dexrazoxane-associated secondary AML are limited and conflicting. We sought to estimate the secondary AML risk in children receiving dexrazoxane after anthracycline exposure.
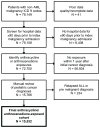
Procedure: A retrospective cohort of children with newly identified malignancies (excluding AML) receiving anthracyclines between January 1, 1999 and March 31, 2011 was established using the Pediatric Health Information System (PHIS). Patients were followed for all subsequent admissions to identify dexrazoxane exposures and secondary AML, defined by AML ICD-9 codes and AML induction chemotherapy. Logistic regression was used to model the association of dexrazoxane and secondary AML risk. A propensity score was used to adjust for measurable confounding.
Results: Of 15,532 patients in the cohort exposed to anthracyclines, 1,406 received dexrazoxane. The secondary AML rate was 0.21% (3 of 1,046) in dexrazoxane-exposed and 0.55% (77 of 14,126) in unexposed patients. In a propensity score-adjusted multivariate analysis, dexrazoxane exposure was not associated with an increased risk of secondary AML, OR = 0.38, 95% CI 0.11-1.26.
Conclusions: Dexrazoxane was not associated with an increased risk of secondary AML in a large cohort of pediatric cancer patients receiving anthracyclines in US hospitals. While these data support dexrazoxane's safety in the general pediatric oncology population, additional studies are needed to confirm these findings and to quantify dexrazoxane's long-term cardioprotective effects.
Keywords: cardiotoxicity after cancer therapy; dexrazoxane; epidemiology; secondary malignancy.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Author Brian T. Fisher receives research support from Pfizer Pharmaceuticals. All other authors declare that they have no conflicts of interest.
Figures
References
-
- Ginsberg JP, Cnaan A, Zhao H, et al. Using health-related quality of life measures to predict cardiac function in survivors exposed to anthracyclines. J Clin Oncol. 2004;22:3149–3155. - PubMed
-
- van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. - PubMed
-
- Steinherz LJ, Steinherz PG, Tan CT, et al. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. - PubMed
-
- Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

