Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps
- PMID: 24670033
- PMCID: PMC4123211
- DOI: 10.1517/14728222.2014.902938
Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps
Abstract
Introduction: Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the acute onset of noncardiac respiratory insufficiency associated with bilateral lung infiltrations. During the past decade, mechanical ventilation strategies using low tidal volumes have reduced the mortality of ALI/ARDS to ∼ 20 - 40%. However, ALI/ARDS continues to be a major factor in global burden of diseases, with no pharmacological agents currently available.
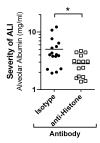
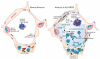
Areas covered: In this review, we discuss several inflammatory proteins involved in the molecular pathogenesis of ALI/ARDS. The complement cleavage product, C5a, is a peptide acting as a potent anaphylatoxin. C5a may trigger the formation of neutrophil extracellular traps (NETs) and release of histone proteins to the extracellular compartment during ALI/ARDS. NETs may activate platelets to release TGF-β, which is involved in tissue remodeling during the later phases of ALI/ARDS. Interception of C5a signaling or blockade of extracellular histones has recently shown promising beneficial effects in small animal models of ALI/ARDS.
Expert opinion: Novel protein-based strategies for the treatment of ALI/ARDS may inspire the hopes of scientists, clinicians, and patients. Although neutralization of extracellular histones/NETs, C5a, and TGF-β is effective in experimental models of ALI/ARDS, controlled clinical trials will be necessary for further evaluation in future.
Keywords: C5a; TGF-β; antibody; extracellular histones; inflammation; neutrophil extracellular traps.
Figures

References
-
- Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526–33. - PubMed
-
- Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Critical care medicine. 2003;31(6):1607–11. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–93. - PubMed
-
-
Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301–8. NoAuthorsListed. * This clinical study was a milestone to improve the clinical management of ALI/ARDS by simple reduction of tidal volumes.
-
-
- National Heart L. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
